Contents
Legal Notice ..........................................................................4
Introduction to this Manual ................................................... 5
Scope .........................................................................6
About the safety notices in this document .................. 7
Disclaimer ................................................................. 8
Introduction to Agfa CR Plates and Cassettes ......................... 9
Intended Use ............................................................10
Intended User .......................................................... 11
System Documentation ............................................12
Product Complaints ................................................. 13
Compliance ..............................................................14
Design ......................................................... 15
Labeling .......................................................15
Environmental Protection ........................................ 16
CR Plate ....................................................... 17
CR Cassette ..................................................18
Safety Directions ......................................................19
General Safety Instructions .......................... 19
Using the Agfa CR Plates and Cassettes ................................ 20
CR MD1.0 Cassettes ................................................. 21
Large format CR General cassette ................22
Small format CR General cassette ................ 24
Image Plate ..................................................26
CR HD5.0S General Detector ................................... 27
Orientation of large format CR General cassette .......29
Orientation of small format CR General cassette ...... 30
CR Full Leg Full Spine (FLFS) cassette ......................31
CR MD1.0F General Image Plate and Cassette ..........32
Exposure outside the cassette .......................33
Performing an examination ......................... 34
Compatibility ...............................................35
Cleaning the image plate adapter .................36
CR DD1.0 Vet Image Plate and Cassette ....................37
Exposure outside the cassette .......................39
Performing an examination ......................... 40
Black border and cropping on NX .................42
Cleaning the image plate adapter .................43
Precautionary Measures .......................................... 44
First Use and Normal Operation ...................45
Transport .....................................................45
Storage ........................................................ 45
Maximum Cassette Load .............................. 45
Handling the Image Plate .............................46
Cleaning ...................................................... 46
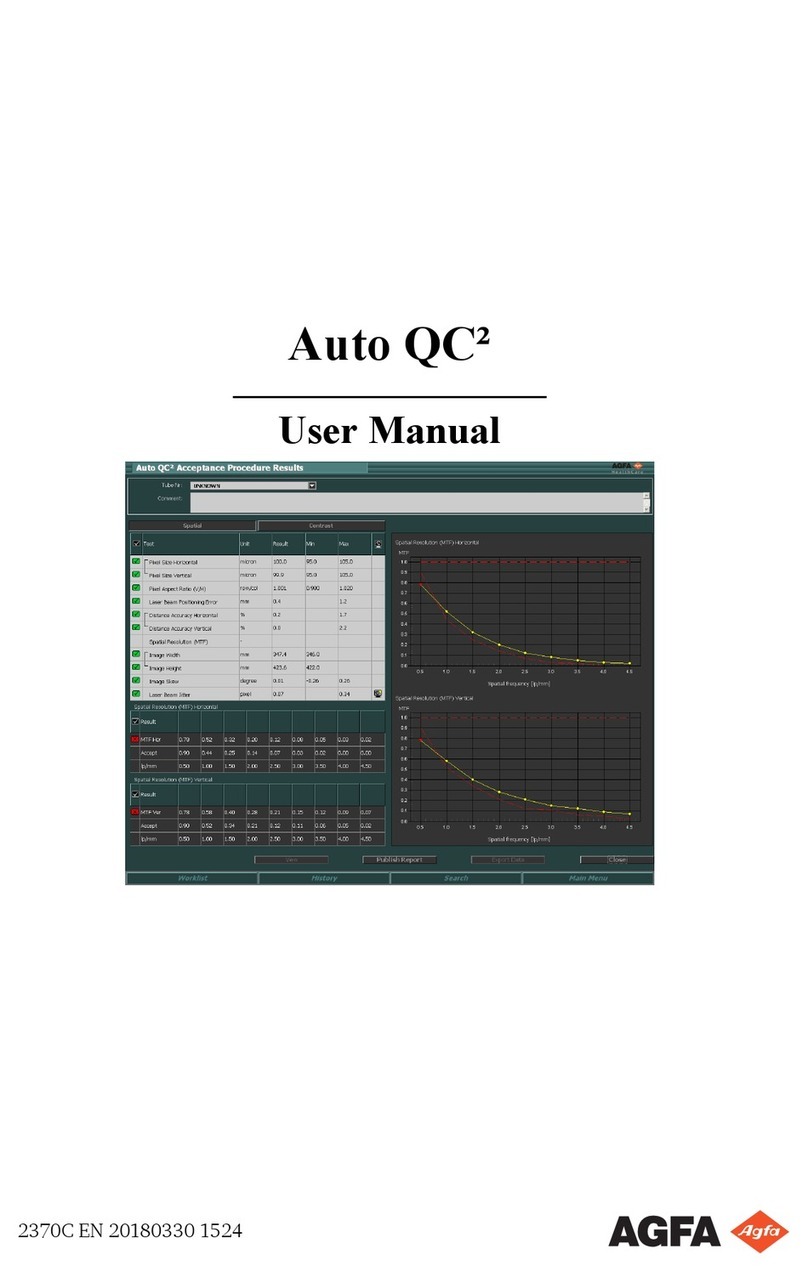
Quality Control ............................................52
ii | AGFA CR Plates and Cassettes | Contents
2492F EN 20180417 1049