Bio-Tek Epoch 2 User manual

Epoch 2
Microplate Spectrophotometer
INSTRUCTIONS FOR USE
BioTek Instruments, Inc.
1771011 Revision A

Preface Page 2 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Contents
Installation............................................................................................................ 12
Getting Started..................................................................................................... 17
Maintenance ........................................................................................................ 33
Qualification ......................................................
Specifications........................................................................................................ 43
....................................................39

Preface Page 3 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Preface
Notices
BioTek® Instruments, Inc.
100 Tigan Street
Highland Park, P.O. Box 998
Winooski, Vermont 05404-0998 USA
All Rights Reserved
© 2018, BioTek®Instruments, Incorporated. No part of this publication may be
reproduced, transcribed, or transmitted in any form, or by any means electronic or
mechanical, including photocopying and recording, for any purpose other than the
purchaser’s use without written permission of BioTek Instruments, Inc.
Trademarks
BioTek®is a registered trademark, and Epoch™, Take3™, BioStack™, and Gen5™ are
trademarks of BioTek Instruments, Inc. BioCell™ is a trademark of BioTek Instruments
and is patented under U.S. patent number 5,963,318.
Microsoft®, Windows®, and Excel®are either registered trademarks or trademarks of
Microsoft Corporation in the United States and/or other countries.
All other trademarks are the property of their respective holders.
Restrictions and Liabilities
Information in this document is subject to change and does not represent a
commitment by BioTek Instruments, Inc. Changes made to the information in this
document will be incorporated in new editions of the publication. No responsibility is
assumed by BioTek for the use or reliability of software or equipment that is not
supplied by BioTek or its affiliated dealers.

Preface Page 4 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Contact Information
Global Service and Support
Instrument service and repair is available worldwide
www.biotek.com/service_support
U.S. Headquarters
BioTek® Instruments, Inc.
100 Tigan Street
Highland Park, P.O. Box 998
Winooski, Vermont 05404-0998 USA
www.biotek.com
European Coordination Center/Authorized European Representative
BioTek® Instruments GmbH
Kocherwaldstrasse 34
D-74177 Bad Friedrichshall
Germany
www.biotek.de
Phone: +49 (0) 7136 9680
Fax: +49 (0) 7136 968 111
Email: [email protected]
Instructions for Use Requirements
This document fulfills the basic needs of persons operating this device, according to
the requirements of the In Vitro Diagnostic Directive (98/79/EC) for “Instructions for
Use.” Some of the device's higher-level functions and features, as well as certain
detailed maintenance and qualification routines, are described in the operator’s
manual.
Intended Use Statement
This instrument is intended for IVD use. The performance characteristics of the data
reduction software have not been established with any laboratory diagnostic assay.
Users must evaluate this instrument and PC-based software in conjunction with their
specific assay(s). This evaluation must include the confirmation that performance
characteristics for the specific assay(s) are met.

Preface Page 5 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Quality Control
It is considered good laboratory practice to run laboratory samples according to
instructions and specific recommendations included in the assay package insert for the
test to be conducted. Failure to conduct Quality Control checks could result in
erroneous test data.
Warnings
Operate the instrument on a level, stable surface away from excessive humidity.
Bright sunlight or strong incandescent light can reduce the linear performance
range of the instrument.
Measurement values may be affected by extraneous particles in the microplate
wells. A clean work area is necessary to ensure accurate readings.
When operated in a safe environment according to the instructions in this
document, there are no known hazards associated with the instrument.
However, the operator should be aware of certain situations that could result in
serious injury; these may vary depending on the instrument model. See Hazards
and Precautions.
Hazards
The following hazard warnings are provided to help avoid injury:
Warning! Internal Voltage. Always turn off the power switch and unplug the
power supply before cleaning the outer surface of the instrument.
Warning! Power Rating. The instrument’s power supply or power cord must
be connected to a power receptacle that provides voltage and current within
the specified rating for the system. Use of an incompatible power receptacle
may produce electrical shock and fire hazards.
Warning! Electrical Grounding. Never use a plug adapter to connect primary
power to the external power supply. Use of an adapter disconnects the utility
ground, creating a severe shock hazard. Always connect the power cord
directly to an appropriate receptacle with a functional ground.
Warning! Service. Only qualified technical personnel should perform service
procedures on internal components.
Warning! Accessories. Only accessories that meet the manufacturer's
specifications shall be used with the instrument.

Preface Page 6 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Warning! Lubricants. Do not apply lubricants to the microplate carrier or
carrier track. Lubricant on the carrier mechanism or components in the carrier
compartment will attract dust and other particles, which may obstruct the
carrier path and cause the instrument to produce an error.
Warning! Liquids. Avoid spilling liquids on the instrument; fluid seepage into
internal components creates a potential for shock hazard. If a spill occurs while
a program is running, abort the program and turn off the instrument. Wipe up
all spills immediately. Do not operate the instrument if internal components
have been exposed to fluid. Contact BioTek Technical Assistance Center for
assistance.
Warning! Unspecified Use. Failure to operate the equipment according to the
guidelines and safeguards specified in this manual could result in a hazardous
condition.
Warning! Software Quality Control. The operator must follow the
manufacturer’s assay package insert when modifying software parameters and
establishing reading methods. Failure to conduct quality control checks could
result in erroneous test data.
Warning! Reader Data Reduction Protocol. No limits are applied to the raw
measurement data. All information displayed on the screen, sent to an
attached printer, or exported via computer control must be thoroughly
analyzed by the operator.
Warning! Potential Biohazards. Some assays or specimens may pose a
biohazard. This hazard is noted by the symbol shown here. Adequate safety
precautions should be taken as outlined in the assay’s package insert. Always
wear safety glasses and appropriate protective equipment, such as chemical-
resistant rubber gloves and apron.
Precautions
The following precautions are provided to help avoid damage to the instrument:
Caution: Service. The instrument should be serviced by BioTek-authorized
service personnel. Only qualified technical personnel should perform service
procedures on internal components.
Caution: Spare Parts.Only approved spare parts should be used for
maintenance. The use of unapproved spare parts and accessories may result in
a loss of warranty and potentially impair instrument performance or cause
damage to the instrument.
Caution: Environmental Conditions. Do not expose the system to temperature
extremes. For proper operation, the temperature near the instrument should
remain within the range listed in Specifications. Performance may be
adversely affected if temperatures fluctuate above or below this range.

Preface Page 7 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Caution: Sodium Hypochlorite. Do not expose any part of the instrument to
the recommended diluted sodium hypochlorite solution for more than 20
minutes. Prolonged contact may damage the instrument surfaces. Be certain
to rinse and thoroughly wipe all surfaces.
Caution: Power Supply. Use only the power supply shipped with the
instrument. Operate this power supply within the range of line voltages listed
on it.
Caution: Shipping Hardware. The shipping hardware must be removed before
operating the instrument and reinstalled before repackaging the instrument
for shipment.
Caution: Disposal. Dispose of the instrument according to Directive
2012/19/EU, “on waste electrical and electronic equipment (WEEE)” or local
ordinances.
Caution: Warranty. Failure to follow maintenance protocols may void the
warranty. See Maintenance.
Caution: Electromagnetic Environment. Per IEC 61326-2-6 it is the user’s
responsibility to ensure that a compatible electromagnetic environment for
this instrument is provided and maintained in order that the device will
perform as intended.
Caution: Electromagnetic Compatibility. Do not use this device in close
proximity to sources of strong electromagnetic radiation (e.g., unshielded
intentional RF sources), because these may interfere with the proper
operation.
Caution: Touchscreen. Do not use sharp implements to operate the
touchscreen.

Preface Page 8 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
CE Mark
Directive 2014/30/EU: Electromagnetic Compatibility
Emissions—Class A
The system has been type-tested by an independent, accredited testing laboratory and
found to meet the requirements of EN 61326-1: Class A for Radiated Emissions and
Line Conducted Emissions.
Verification of compliance was conducted to the limits and methods of EN 55011 –
(CISPR 11) Class A. In a domestic environment it may cause radio interference, in which
case you may need to mitigate the interference.
Immunity
The system has been type-tested by an independent, accredited testing laboratory and
found to meet the requirements of EN 61326-1 and EN 61326-2-6 for Immunity.
Verification of compliance was conducted to the limits and methods of the following:
EN 61000-4-2, Electrostatic Discharge
EN 61000-4-3, Radiated Radio Frequency (RF) Immunity
EN 61000-4-4, Electrical Fast Transient/Burst Immunity
EN 61000-4-5, Surge Immunity
EN 61000-4-6, Conducted RF Disturbance Immunity
EN 61000-4-11, Voltage Dips, Interruptions and Short Variations
Directive 2014/35/EU Low Voltage (Safety)
The system has been type-tested by an independent testing laboratory and was found
to meet the requirements of this Directive. Verification of compliance was conducted
to the limits and methods of the following:
EN 61010-1. "Safety requirement for electrical equipment for measurement, control
and laboratory use. Part 1, General requirements."
EN 61010-2-010. "Particular requirements for laboratory equipment for the heating of
materials."
Directive 2012/19/EU: Waste Electrical and Electronic Equipment
Disposal Notice: Dispose of the instrument according to Directive 2012/19/EU, “on
waste electrical and electronic equipment (WEEE)” or local ordinances.

Preface Page 9 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Electromagnetic Interference and Susceptibility
Canadian Department of Communications Class A: This digital apparatus does not
exceed Class A limits for radio emissions from digital apparatus set out in the Radio
Interference Regulations of the Canada Department of Communications.
User Safety
This device has been type-tested by an independent laboratory and found to meet the
requirements of the following:
•Canadian Standards Association CAN/CSA C22.2 No. 61010-1, “Safety
requirements for electrical equipment for measurement, control and laboratory
use; Part 1: General requirements.”
•EN 61010 Standards, see CE Mark starting on page 8.
Preface Page 10 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
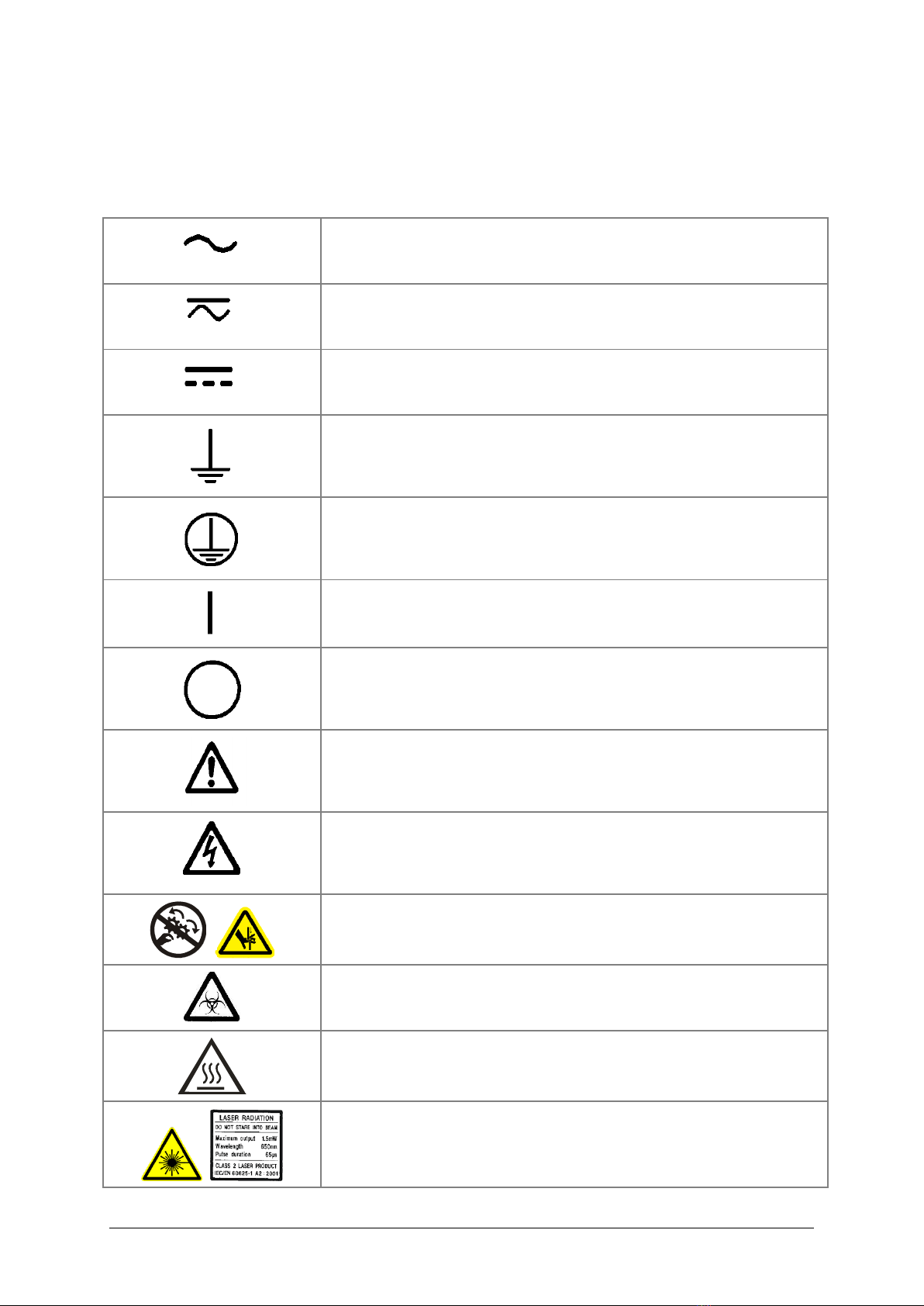
Safety Symbols
Some of these symbols appear on the instrument or accessories:
Alternating current
Both direct and alternating current
Direct current
Earth ground terminal
Protective conductor terminal
On (Supply)
Off (Supply)
Caution (refer to accompanying documents)
Warning, risk of electric shock
Warning, risk of crushing or pinching
Warning, potential biohazards
Warning, hot surface
Laser radiation: Do not stare into beam

Preface Page 11 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Laser energy: Exposure near aperture may cause burns. Do not
stare directly at the laser during operation.
In vitro diagnostic medical device
Separate collection for electrical and electronic equipment
Consult instructions for use
Installation Page 12 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Installation
Models
Model
Touchscreen
Cuvette Port
EPOCH2NS
EPOCH2NSC
•
EPOCH2TS
•
EPOCH2TSC
•
•
Package Contents
Item
Notes
A specific model of an Epoch 2 instrument
per sales order
All required accessories to power the
instrument
Interface cables, if required
User manual and/or instructions
Optional accessories per sales order,
unless shipped separately

Installation Page 13 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Unpack the Box

Installation Page 14 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Remove the Shipping Hardware
Do not turn on the reader before the shipping hardware is removed!
Select an Appropriate Location
Install the reader on a level, stable surface. For models without a touchscreen, select
an area where temperatures between 18°C and 40°C can be maintained. For models
with a touchscreen, the temperature must be between 18°C and 30°C. The reader is
sensitive to extreme environmental conditions. Avoid excessive humidity, excessive
ambient light, and dust.
(Gen5 control only) Prepare the Host Computer
❖Follow instructions in the Gen5 Getting Started Guide to install the software.
(Gen5 control only) Connect the Host Computer and Reader

Installation Page 15 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Install the Power Supply
Install the Cuvette Holder
Models with a cuvette port

Installation Page 16 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Turn on the Reader and Run the Power-Up System Test
Allow the reader to settle at room temperature before you turn it on.
(Gen5 control only) Start Gen5 and Test Communication
1. Start Gen5. If prompted to add a reader, click Yes. Otherwise, select System >
Instrument Configuration > Add Reader.
2. Select Epoch 2 and click OK. The reader name and serial number appear. Click on the
reader name and then click Test Communications. The message, “The reader is
communicating!” should appear.
If the communication attempt is not successful: Make sure the reader is turned on and
the USB cable is secure on both ends. Consult the Gen5 Getting Started Guide
(supplied as a PDF on the Gen5 software USB flash drive) for additional
troubleshooting information.
Repackage the Instrument (if needed)
If the reader has been exposed to potentially hazardous material,
decontaminate it to minimize the risk to all who come in contact with the
reader during shipping, handling, and servicing. Decontamination prior to
shipping is required by the U.S Department of Transportation regulations.
See the Maintenance section for instructions.
Remove any labware from the carrier and, if used cuvette, before shipment.
Spilled fluids can contaminate the optics and damage the instrument.
Replace the shipping hardware before repackaging the reader.
Reverse unpacking instructions.

Getting Started Page 17 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Getting Started
External Components
Touchscreen Models
1. USB port for data
output
2. microplate carrier
access door
3. cuvette holder
(if equipped)
4. power on/off
5. carrier in/out,
status LED
6. USB port for printer
7. power inlet
8. USB port for host
computer
(if used)

Getting Started Page 18 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Non-Touchscreen Models
1. microplate carrier
access door
2. cuvette holder
(if equipped)
3. power on/off
4. carrier in/out,
status LED
5. power inlet
6. USB port for host
computer

Getting Started Page 19 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
Optional Printer
Refer to the printer's manual for installation and setup instructions.
Using the Touchscreen
Use your fingertip to operate the touchscreen. A sharp stylus or pencil will damage
the surface. You can use a stylus designed for resistive touchscreens.
Main Menu
Up to 60 uniquely named protocols can be saved on the reader at one time, excluding
the predefined Take3 protocols.
Protocols created through the touchscreen are limited to a single read step and, for
Endpoint, Kinetic, and Take3 protocols, up to 12 blank wells. Use Gen5 if your assays
require more complex reading methods or plate layouts.
The left side of the Main Menu lists the five most recently used protocols. The tabbed
sections in the middle contain the protocols created for each protocol type, and the
Take3 plate (if used). To run an existing protocol, tap its name, define the wells to be
read if fewer than the full plate, and tap Start.

Getting Started Page 20 of 42
Epoch 2 Instructions for Use 1771011 Revision ABioTek Instruments, Inc.
The right side of the Main Menu provides access to the following features:
•Quick:Define and run an Endpoint read, perform a standalone shake, or
incubate a plate. Quick reads do not support shake or delay actions, and they
read the full plate.
•Protocol:Edit, create (and save), delete, and copy protocols. Through this menu
option, protocols support shake and delay actions.
•Results:Provides access to results for the 12 most recently run protocols. Tap a
protocol name to view its results, and then tap the screen to cycle through the
available data sets (e.g., Raw data, Delta OD, Blanked data).
•Instrument:Run a system test, define date/time settings, enable output to a
printer and/or USB flash drive, configure a Take3 plate, and control
temperature.
Confirm or Set the Time and Date
From the Main Menu, tap Instrument and select the Configuration tab.
•Tap the Time / Time Format and Date / Date Format fields to change the
current settings.
•Set the Decimal Symbol to a period or comma.
•Set the List Separator, used in exported .csv files, to a comma or semicolon.
Table of contents
Other Bio-Tek Laboratory Equipment manuals

Bio-Tek
Bio-Tek Synergy HTX User manual

Bio-Tek
Bio-Tek ELx800 User manual

Bio-Tek
Bio-Tek Synergy H1 User manual

Bio-Tek
Bio-Tek Synergy H1 User manual

Bio-Tek
Bio-Tek ELx50 User manual

Bio-Tek
Bio-Tek 405 TS User manual

Bio-Tek
Bio-Tek MultiFlo FX User manual

Bio-Tek
Bio-Tek Epoch User manual

Bio-Tek
Bio-Tek Synergy LX User manual

Bio-Tek
Bio-Tek 800TSI User manual























