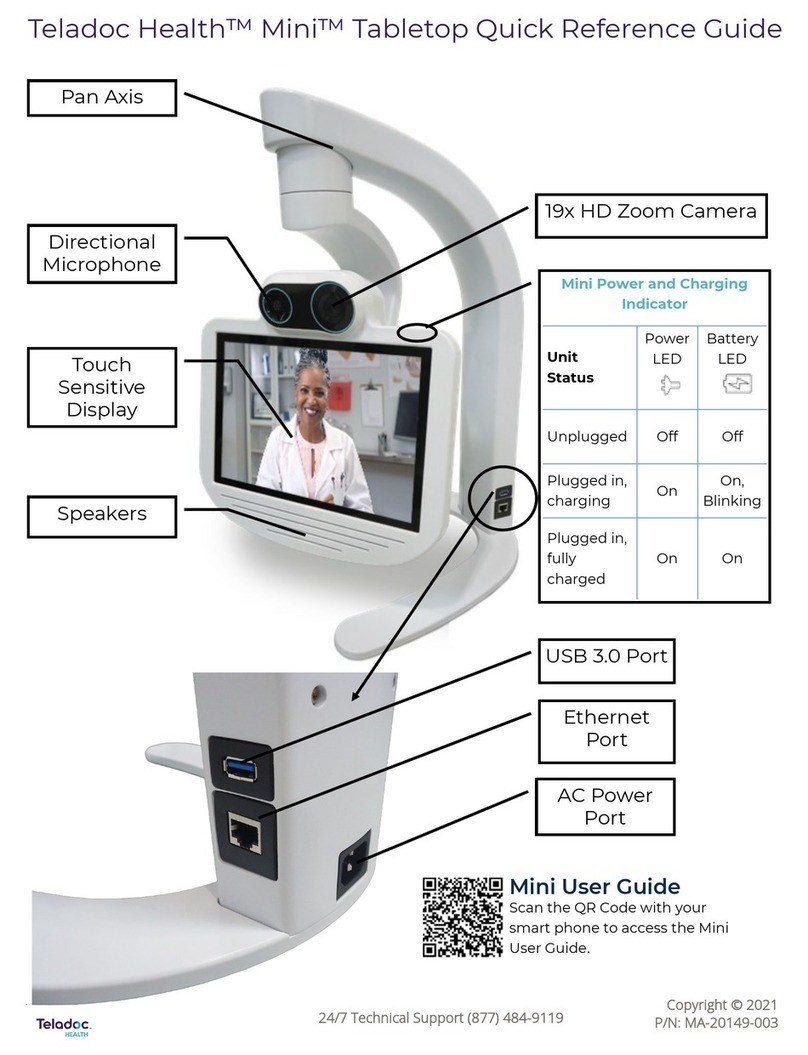
Essilor Instruments Retina 800 User manual

CONTENTS
I. INTRODUCTION 5
II. SAFETY 7
1. Documentation 8
2. Definitions 8
3. Symbols 9
4. Classification 10
a. Technical features 10
b. Environmental conditions 10
5. Indication for use 11
6. Safety requirements 12
a. Contraindications 12
b. Warnings 12
7. Precautions 14
a. Special patient populations 14
b. Adverse reactions 14
c. Prescription devices 14
8. Disclaimer of liability 14
III. SUPPLY PACKAGE 15
1. Packaging and transport 16
2. Unpacking and storage 16
3. Package contents 17
IV. INSTALLATION OF THE DEVICE 19
1. Installation 20
2. Initial powering ON 21
3. First login 21
4. Change password 23
5. Recover a lost password through the PUK code 26
6. Turn ON/OFF the tablet 27
a. Switch ON the tablet and activate the APP 27
b. Switch OFF 30
V. USE OF THE DEVICE 31
1. Enter patient 32
2. Perform an exam 35
3. Applicable features 36
a. [Actions] 37
b. [Filters] 51
c. [Compare images] 56
4. Consult patient records 58
VI. SETTINGS 59
1. Date and time 60
USER MANUAL> CONTENTS

2. Language 61
a. Change system language 61
b. Change APP language 62
c. Change language on the tablet keyboard 62
3. How to set ... 66
a. ... [Retinas sharing] 66
b. ... [PC shared folder] 67
c. ... [DICOM] 72
d. ... AI (Artificial Intelligence) sharing images for software 73
e. ... [Email Sending] 75
4. Remote support (TeamViewer) - Optional 82
5. Cloud access 85
6. Telemedicine 92
7. Retina800 adapter - Optional accessory 96
a. Overview 96
b. Initial configuration 96
c. Transfer of the image 99
VII. MAINTENANCE 103
1. Cleaning the device 104
a. Cleaning the lens 104
b. Cleaning the cheekbone and forehead rests 104
2. Periodic checks 104
VIII. TROUBLESHOOTING 105
IX. ASSISTANCE, NOTIFICATION, ALERT AND LABEL 109
1. Assistance 110
2. Notification & Alert 110
3. ID plate 110
4. Label 110
X. GUIDANCE AND MANUFACTURER'S DECLARATION 111
1. Electromagnetic emission 112
2. Electromagnetic immunity 113
XI. QR CODE 115
USER MANUAL> CONTENTS

I. INTRODUCTION

II. SAFETY

1. DOCUMENTATION
This usage and maintenance manual, together with the instructions on the label, reflects the information
supplied by the manufacturer in accordance with Directive 93/42/EEC amended by 2007/47/EC.
The medical device must be accompanied by the information needed to ensure that it is used safely, taking
the training and knowledge of potential users into account.
The manual is an integral part of the device, and therefore must be maintained with extreme care and
always attached, in the event the product is transferred of to third parties.
It is addressed to operators, the owner, users and maintenance technicians.
The manual provides guidance on the technical characteristics, proper use of the device, transportation,
storage, maintenance, disposal and security precautions.
Any changes to the manufacturer's instructions that prove relevant for patient and/or operator safety will be
promptly communicated to the owners/users of the product through all channels useful for these purposes.
Any other changes and/or additions are excluded from the manufacturer's notification obligation.
If this manual, the device's labels and/or markings are even partially damaged, faded, illegible in part or in
full, an additional copy must be immediately requested from the dealer or manufacturer.
2. DEFINITIONS
The terms referred to in this manual shall have the meaning given below:
Medical device: Device intended by the manufacturer to be used on human beings for care, diagnosis or
the alleviation of disease.
Patient: The subject who undergoes a medical exam with the use of the device.
Operator: It is the person assigned to the use of the device according to the procedures reported in the
intended use.
Retina800 - Fundus camera > V2 - 06-2019
8
USER MANUAL> II. SAFETY

3. SYMBOLS
The use of symbols is seen in this manual and on the device, whose meaning is described in the following
table.
Symbols Meaning
General prohibition
Forbidden operation
Refer to user manual follow the instructions
General mandatory action
Symbol for compliant with CE marking i.e. with applicable European directives
Serial No.
Model
OI ON/OFF switch (mean of isolation from the supply means)
This is the type B equipment
This product complies with EU Directive 2012/19/EC. The crossed bin symbol on the device
indicates that the product must be treated separately from household waste at the end of its
useful life, it must be taken to a different collection point for electrical and electronic
equipment, or returned to the seller when buying a new piece of equivalent equipment. The
user is responsible for transporting the equipment to the appropriate collection facilities at
the end of its life. Proper collection for the subsequent delivery of the device intended for
recycling, treatment and environmentally compatible disposal helps prevent a negative
impact on the environment and human health, and promotes the recycling of the materials
from which the product is made. For more detailed information concerning available
collection systems, contact your local waste disposal service
Symbol for “manufacturer”
Manufacturing date
General warning signal
Pay attention if this signal is shown
Warning: dangerous voltage
Pay attention if this signal is shown
Symbol for prescription only. U.S. Federal law restricts this device to sale by or on the order
of a physician or properly licensed practitioner
Protective earth (ground)
USER MANUAL> II. SAFETY
9
Retina800 - Fundus camera > V2 - 06-2019

4. CLASSIFICATION
Classification
This device is compliant with marking.
Date of first marking: September 2018
Class I medical device
The expected life of the device and its components is 4 years.
a. Technical feat res
Technical data Val e
Supply voltage 100-240 VAC
Rated frequency 50/60 Hz
Internal voltage 24 VDC - 12 VDC - 5 VDC
Maximum absorbed power 52 VA
Maximum absorbed current 400 mA
Safety class I
Applied part Type BF
Dimensions 340 x 430 x 460 mm
Weight 14.7 kg
Sensor resolution 2.1 mpixels
b. Environmental conditions
Temperature Humidity Atmospheric pressure
Use [10°C ; 40°C] Maximum 90 % without condensation [800hPa ; 1060hPa]
Storage [0°C ; 60°C] Maximum 90 % without condensation [700hPa ; 1060hPa]
Transportation [-10°C ; 60°C] Maximum 90 % without condensation [500hPa ; 1060hPa]
Lighting: for a more successful result, it is recommended to perform the exam in a dimly lit area.
The device does not meet the temperat re req irements of ISO 15004-1 for storage. Do not store
the device in conditions where the temperat re may exceed 60°C or drop lower than 0°C.
Retina800 - Fundus camera > V2 - 06-2019
10
USER MANUAL> II. SAFETY

5. INDICATION FOR USE
This device is an automatic eye-fundus camera intended for taking digital images of a human retina without
the use of a mydriatic agent.
This manual has been drawn up taking the operator characteristics, knowledge, education level and training
into account.
Operators using Retina800 must:
•Be adequately trained in the use of the device
•Be informed about the risks and side effects
•Be in possession of the qualifications required by law
•Read and understand every part of the user manual accompanying the
device itself
•Carefully evaluate any contraindications performing an exam
Check the suitability of the environment prior to each use.
The operator is always entirely responsible for compliance and compatibility of the premises in which the
product is used.
Like all electromedical devices, Retina800 requires special precautions as regards electromagnetic
compatibility. The environment in which the device is used must comply with the characteristics set forth in
the electromagnetic compatibility tables in the final section of the present document, and must also be
connected to a compliant electrical system.
It is a class I medical device as in Annex IX to Directive 93/42/EEC amended by 2007/47/EC, is built in
compliance with national and international regulations concerning medical devices.
The Retina800 medical device takes color photographs of the bottom of the retina with a retinal field of 45°.
By means of the tablet, the doctor begins the automatic exam that results in a photo of the patient's retina,
saved in the internal database of the device in .jpeg format. Retina800 is able to store acquired images and
patient data in a local or remote database, through a secure data encryption system that complies with
current privacy regulations.
The exam can be performed on all patients. The successful outcome of the exam (image quality of the
retina) depends on the transparency of the optical channel that spans from the cornea to the retina (for
example, this may be reduced in subjects with cataracts or crystalline lens with low transparency).
The use of this electromedical device on children and minors is prohibited without the assistance of adults.
Retina800 is comprised by a movable head equipped with optics, powered at 24 Vdc by means of an AC/DC
adaptor (provided). To initiate the exam and view the image of the retina, a user interface is provided by a
tablet (also included).
The device is only intended for exam of the retina as indicated herein.
USER MANUAL> II. SAFETY
11
Retina800 - Fundus camera > V2 - 06-2019

It must be used within the limits and according to the procedures explicitly described by the manufacturer in
this manual. The manufacturer is therefore deemed to be held harmless for all liability for damages resulting
from misuse of the product by untrained persons, as well as any unauthorized modifications or interventions,
including the use of parts other than those supplied directly by Essilor (or its authorized parties), exceptional
events and the total or partial non-observance of the instructions in this manual.
Where the device can be sed
The device can be used in hospitals, public and private health clinics and in public and private environments
where eye or systemic and/or vision tests are carried out. The device can be used by medical personnel in
collaboration with healthcare professionals and/or optometry professionals. Users must be adequately
trained in the use of the device and informed about the risks and side effects and possess the qualifications
required by law. The device, for the sole function of image acquisition and excluding the reporting (and
clinical interpretation) part, can be used in self-acquisition mode by an appropriately trained person or
assisted by appropriately trained personnel.
6. SAFETY REQUIREMENTS
a. Contraindications
No contraindication.
b. Warnings
This device is to be used for its intended use, according to the indicated instructions and directions for use.
Essilor does not assume responsibility for damage to persons or property caused by improper use and/or
misuse of the device, or for its use other than the function for which it was intended by its manufacturer.
The use of Retina800 assumes that the operator has knowledge of this manual and its user guide, as well as
awareness of the risks related to improper use and misuse.
Therefore, it must not be used by persons who do not have adequate knowledge of the device and its
methods/features of use, with the caveat that in the event of any questions and/or uncertainties about its
operation and use, the operator will contact the authorized dealer and/or the manufacturer directly in order
to acquire any clarifications and/or explanations, or, where necessary and expressly requested, specific
assistance in the terms and procedures provided for in the receipts of purchase.
Carefully read this manual and warnings before use the device.
The following must not be carried out, as they may compromise the compliance and/or the characteristics of
the device:
•Incorrect installation
•Improper use
•Use of parts and/or third-party accessories that are not approved by the manufacturer
•Interventions and/or tampering by unauthorized personnel
•Lack of or improper maintenance
Retina800 - Fundus camera > V2 - 06-2019
12
USER MANUAL> II. SAFETY

The warnings to be observed during installation, operation and maintenance of the
device are contained below, in order to ensure the fulfilment of the requirements
for operator and end-user safety as well as the proper functioning of the device.
•Any manipulation, replacement or operation performed on the device that is not carried out by Essilor
personnel authorized shall void the warranty and exempt the manufacturer from any liability for direct
and/or indirect damages that might be caused to persons or property
•Use the power cord supplied with the product. Periodically check the integrity of the cable. Fully insert
the plug into the mains socket on the rear side of the device
•Use a power supply voltage between 100-240VAC 50/60Hz (not different from that listed on the
plate)
•To avoid danger to persons or property, observe all nominal data and markings on the product.
Consult the manual before making connections to the device
•Avoid exposed circuitry. Do not touch exposed connections or components connected to power supply
•Do not operate in the event of suspected fault or if cracks are present on the casing
•If you suspect that the device is faulty and/or damaged, have it checked by specialized and approved
personnel of the manufacturer
•Avoid contact or penetration of liquids or powders into the device
•Do not operate in a potentially explosive atmosphere and/or in the presence of flammable mixtures
•Do not use Retina800 outdoors. It was designed and built for use in areas that are closed and
protected from the elements
•Use the device only with the original spare parts supplied by the manufacturer
•Make sure that the features of the electric network comply with the power requirements of the device
as indicated on its label and in this manual
•Do not use devices other than Retina800 simultaneously on the patient
•Do not use the device in environments with high electromagnetic fields that could cause Retina800
and other equipment in the surrounding area to malfunction. Do not keep mobile phones in the
treatment area
•The appliance must be installed and commissioned according to the EMC information contained in this
manual
•Portable and mobile radio communications equipment can affect the operation of the device
•Do not operate near (within 1 m) of a device for short wave or microwave therapy
•The use of accessories other than those supplied may adversely affect the electromagnetic
compatibility performance of Retina800
•Do not simultaneously connect the patient to a high frequency electrosurgical device
•Caution: the use of controls and adjustments or the performance of procedures other than those
specified herein may result in injury to the patient and the operator
•It is recommended to use the device in dim room
Wifi
The minimum Wifi protocol requirements should be WPA-PSK o WPA2. It is excluded free network (without
password) or WEP protocol because that are not safe against unauthorized access, malicious intent, and
cyber-security threats.
The tablet is compatible with 2.4 GHz Wifi.
USER MANUAL> II. SAFETY
13
Retina800 - Fundus camera > V2 - 06-2019

7. PRECAUTIONS
Retina800 may only be used by personnel trained to operate the device, and it is
essential to carefully consider the following precautions.
•The device must only be operated and used by personnel trained on its usage techniques
•To prevent misuse by unauthorized personnel, the operator must log out of the management app at
the end of each exam
•Perform cleaning and maintenance only after disconnecting the device from the mains and switching it
off
•Perform maintenance of the device according to that indicated by the manufacturer
a. Special patient pop lations
The use of this electromedical device on children and minors is prohibited without the assistance of adults.
b. Adverse reactions
No adverse reactions.
c. Prescription devices
Caution: Federal (USA) laws restrict this device to sale by or on the order of a
physician or a properly licensed practitioner.
The clinical interpretation of the images acquired by Retina800 is restricted to licensed eye care
practitioners. The process of making a diagnosis using Retina800 results is the responsibility of the eye care
practitioner. A device specific training is required for any operator to become able to use the system.
8. DISCLAIMER OF LIABILITY
The manufacturer assumes no liability for damages, accidents or injuries caused by failure to follow the
requirements, directions and safety guidelines provided for in this manual.
Essilor will not cover any damages that may result from improper use and/or misuse of the product, nor will
it respond in any way for any damage that may result from wear and tear, negligence, neglect,
manipulation, incorrect/improper installation and/or connection of the products, or by improper use and/or
misuse by the operator/end user of any third parties not authorized to use the device.
Retina800 - Fundus camera > V2 - 06-2019
14
USER MANUAL> II. SAFETY

III. SUPPLY PACKAGE

1. PACKAGING AND TRANSPORT
The device is contained in a cardboard box and adequately protected against vibration arising from a regular
transport by means of specially shaped polyethylene foam. The box must be transported while maintaining it
in the vertical position and avoiding bumps or jerks, and according to the environmental conditions of
transport contained on the packaging.
Verify the integrity of the packaging upon receipt. If any damage to the packaging is noted, the operator
who carried out the shipment must be immediately notified.
Before operating a device whose packaging shows signs of damage, the device itself
must be checked by technical assistance or personnel approved by Essilor.
•If damage caused by transport is noted upon receipt of the device, the device must not be used and
technical assistance must be contacted for an audit and revision of the device itself.
•The user must be fully aware of the contents of the manual and symbols.
2. UNPACKING AND STORAGE
It is necessary that the person unpacking the box has been trained as regards the
risks of such operations.
To properly lift the box without jeopardizing the spine:
•Keep the back straight
•Keep the trunk upright
•Assume a squat position
•The weight lifted should be kept as close to the body as possible
Do not store the products above shoulder height.
The device must be stored in an area that complies with the environmental conditions for storage, as
displayed on its packaging.
Bring the box to an area that is suitable for the extraction and installation of the machine's parts.
Open the package without the use of sharp objects that can damage its content.
Retain the packaging for reuse in the event of that the device needs to be shipped (such as for technical
assistance), as the original packaging provides safe transportation.
Retina800 - Fundus camera > V2 - 06-2019
16
USER MANUAL> III. SUPPLY PACKAGE

3. PACKAGE CONTENTS
Retina800 package contains the following items.
Device
Tablet - Lenovo yoga with charger and micro USB
cable
> The tablet can store about 50000 images.
Mean well AC/DC adaptor GSM60A24 model
Detachable power cord
Lens cap
User manual
USER MANUAL> III. SUPPLY PACKAGE
17
Retina800 - Fundus camera > V2 - 06-2019

Optional accessory
Fundus adapter
Head
With:
1. Forehead rest*
2. Lens
3. Moveable head
4. Cheekbone rests*
*: Applied parts
Socket panel
1. LAN port
2. USB port
3. Battery connection port
4. ON/OFF button
Retina800 - Fundus camera > V2 - 06-2019
18
USER MANUAL> III. SUPPLY PACKAGE

IV. INSTALLATION OF THE DEVICE

1. INSTALLATION
Check the device for damage or dents that may have occurred during transport. If in doubt, contact your
dealer or the manufacturer.
Place the contents of the box on a flat surface and check that all of the components are present and in
good condition.
Connect the plug of the AC/DC adaptor into the socket on the base of Retina800 device.
Turn ON the tablet.
If it does not turn ON, use the special charger and micro USB cable provided in its box.
Connect the AC/DC adaptor plug to a 100-240VAC, 50 / 60 Hz power supply.
Or, that appropriate for the power requirements specified on the nameplate.
Press the power button (for about 3 seconds) until the corresponding green light comes on.
1
2
3
4
5
Retina800 - Fundus camera > V2 - 06-2019
20
USER MANUAL> IV. INSTALLATION OF THE DEVICE

2. INITIAL POWERING ON
Ensure that Retina800 is correctly mounted.
When powering ON for the first time, perform a device configuration. If there is a wireless network, enter the
SSID and password; otherwise enter a SIM card enabled for traffic on the tablet.
Safety and patient well-being are the top priority during the use of Retina800. Follow
the safety indications at the beginning of this manual, as well as all recommendations
provided during the description of the device.
3. FIRST LOGIN
Before you can use the device, a first connection procedure must be performed. It consists of creating an
account (username and password), which will be required when turning on the tablet.
•[Username]: SerialN[email protected]
•[Password]: password
This password is temporary and you will be prompted to change it upon the first
connection.
Go to the website https://retina800.essilor.com
The following screen appears:
Fill in the fields.
Reminder:
•[Username]: SerialN[email protected]
•[Password]: password
>
1
2
USER MANUAL> IV. INSTALLATION OF THE DEVICE
21
Retina800 - Fundus camera > V2 - 06-2019
Other Essilor Instruments Medical Equipment manuals
Essilor Instruments
Essilor Instruments AKR 800 User manual

Essilor Instruments
Essilor Instruments APH 550 User manual
Essilor Instruments
Essilor Instruments Vision-C 600 User manual

Essilor Instruments
Essilor Instruments DELTA User manual
Essilor Instruments
Essilor Instruments Vision-R 800 User manual
Essilor Instruments
Essilor Instruments Mr Blue 2.0 Sun and Sport Edition User manual

Essilor Instruments
Essilor Instruments VISIOSMART 500 User manual
Essilor Instruments
Essilor Instruments EyeViz 300 User manual

Essilor Instruments
Essilor Instruments VISION-R 700 User manual

Essilor Instruments
Essilor Instruments Retina 550 User manual