4400-0108 enFlow Preventive Maintenance Record Rev. C Apr-2011 Page 4 of 8
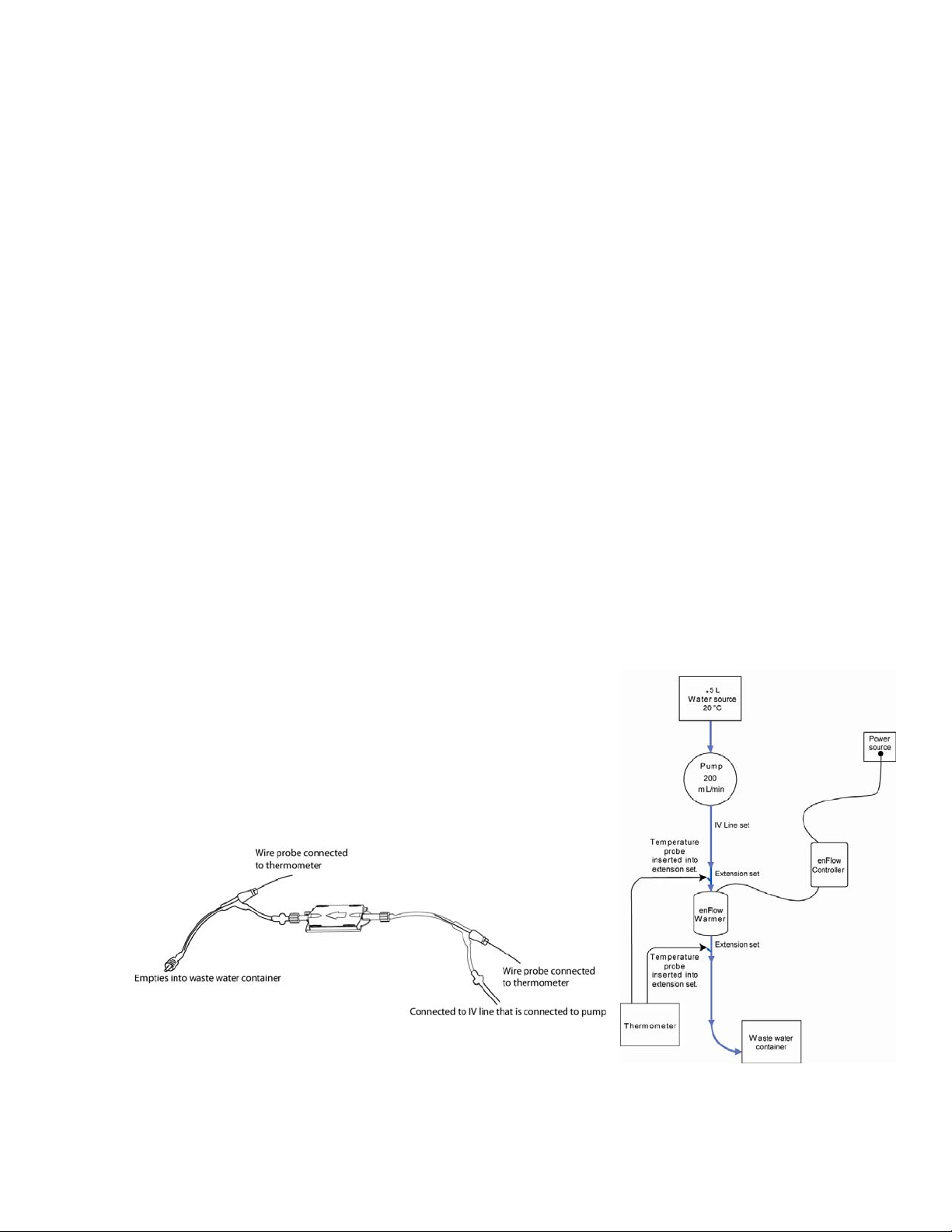
wire is inserted into the tubing just be certain that the wire is in contact with the fluid.
5. Next, put a non-venting cap on the open end of the extension set.
6. Place the cartridge setup in the warmer.
7. Connect the ECG lead from the leakage tester to the wire inserted into the extension set
tubing.
8. Perform the “ECG” lead leakage test.
Functional Tests
1. enCheck Model 400
Equipment
enCheck (Includes “K” type probe.)
Thermal thermocouple meter with ± 5 °C accuracy
enFlow Controller/AC Power Pack
enFlow Warmer
Purpose
The enCheck Tester was developed to quickly and reliably trigger the over-temperature alarm
condition on the enFlow Warmer. Within seconds, the enCheck unit will heat the Warmer to an over-
temperature scenario causing the alarm to sound. Additionally, the enCheck is designed to verify the
Warmer operation at the enFlow’s installation site.
Procedure
A. Normal Mode - Simulated Use Performance Testing
When the enCheck is connected and running in the normal mode, the heat is generated from the
warmer unit using the same technology as when a cartridge is installed. This mode allows for
confirmation of the temperature output of the Warmer.
1. Plug the Controller/AC Power Pack into a hospital grade outlet.
2. Connect the enCheck to the Controller/AC Power Pack by inserting the male plug end of the
enCheck Hubbell connector into the female receptacle on the front face of the Controller/AC
Power Pack. Push it in so that the plug cover is tight against the receptacle.
3. Next, connect the Warmer to the enCheck by inserting the male plug end of the Warmer into
the enCheck female receptacle.
4. Take the temperature probe connector on the enCheck and insert into a thermometer. Set
thermometer to “K” type setting.
5. Insert the enCheck unit into the Warmer. The end of the unit is keyed similar to the Cartridge
so it will only fit in the correct orientation. Close the covers.
6. Confirm the enCheck is set to the normal mode.
7. Move the MAINS power switch on the back of the Controller/AC Power Pack to the ON
position. Wait for the thermometer to stabilize, ≈30 to 60 sec. assuming all equipment is
close to 20 °C.
8. The temperature on the thermometer should be 40 °C ± 2 °C.
B. Overheat Mode – Alarm Test
When operated in the overheat mode, the heat is generated from the contact plate on the underside
of the enCheck unit. The enCheck heats the Warmer to > 45 °C. This temperature range simulates an
over-temperature situation demonstrating the functionality of the alarm (audible, LED’s, and display
(Controller only).
1. Setup is the same as in the above steps 1-7 with the exception of step 4. Skip step 4. (In the
Overheat mode, it is only important to review the temperature of the Warmer, which is
Current Normal
Single Fault
Earth leakage 5 mA 10 mA
Touch/chassis leakage 100 µA 500 µA
Patient leakage 100 µA 500 µA
From Table I. Leakage current limits (from IEC 60601-1).