4 0113103 Notice d'utilisation / User manual / 사용자 매뉴얼
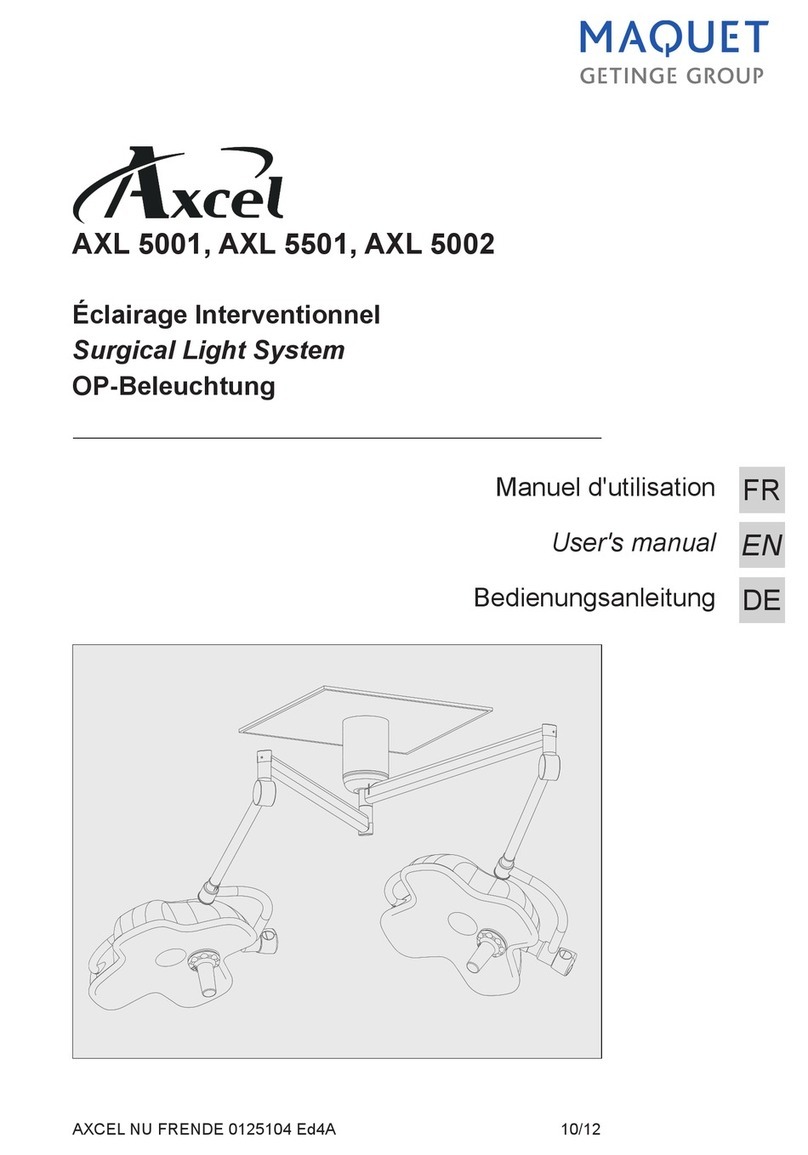
Éclairage opératoire
Surgical light
수술용 조명장치
품질 인증
MAQUET SA 품질시스템 인증서
LNE/G-MED 인증 마크는 수술조명장치의
고안, 제작, 판매, 설치 및 애프터 서비스를
위하여 MAQUET SA사가 자체 개발한 품질
시스템이 국제 규격의 요구사항에 적합하다
는 것을 증명합니다.
- ISO 9001:2000
- NF EN ISO 13485:2004
PRISMALIX 은 다음의 실행 규격을
충족시키도록 설계되었다:
• EN ISO 14971:2000
Medical devices - Application of risk
management to medical devices
(ISO 14971:2000)
•ENISO14971:2000/A1:2003
•EN60601-1:1990
Medical electrical equipment -
Part 1: General requirements for safety
Amendment A1:1993 to EN 60601-1:1990
Amendment A2:1995 to EN 60601-1:1990
Amendment A13:1996 to EN
60601-1:1990
•EN60601-1-2:2001
Medical electrical equipment -
Part 1-2: General requirements for safety
- Collateral standard: Electromagnetic
compatibility - Requirements and tests
•EN60601-1-4:1996
Medical electrical equipment -
Part 1-4: General requirements for safety
- Collateral standard: Programmable
electrical medical systems
Amendment A1:1999 to EN
60601-1-4:1996
•EN60601-1-6:2004
Medical electrical equipment -
Part 1-6: General requirements for safety -
Collateral standard: Usability
•EN60601-2-41:2000
Medical electrical equipment -
Part 2-41: Particular requirements for
the safety of surgical luminaires and
luminaires for diagnosis
이 제품은 다음 보완 기준에 따라 확인을
받았습니다:
CAN/CSA-C22.2 N° 601.1-M90 (R2005) (
캐나다의 경우 국가적 차이 포함)
EN 60601-1:1990+A1 :1993+A2 :1995+A13
:1996, UL 60601-1, 제 1회, 2006-04-26 (
미국의 경우 국가적 차이 포함).
CE 마크
의료기기 관련 1994년 6월 14일자 93/42/
CEE 지침의 요구사항에 부합하는지가 별첨 7
에 따라 평가되었다. 이들 수술 조명은 93/42/
CEE 지침의 별첨 9에 따라 제 1등급에 속한다.
사용 또는 환경이 변경된 경우에
는, MAQUET SA에 연락하여야
제품의 품질을 보증받을 수 있다.
Conformité aux normes
de qualité
Certicationdusystèmequalitéde
MAQUET SA
Le LNE/G-MED certifie que le système
qualité développé par MAQUET SA pour la
conception, la réalisation, la vente, l’instal-
lation et le service après-vente d’éclairages
opératoires est conforme aux exigences des
normes internationales :
- ISO 9001 version 2000
- NF EN ISO 13485 version 2004
L’éclairage opératoire PRISMALIX a été
conçu pour répondre aux normes suivan-
tes:
• EN ISO 14971:2000
Medical devices - Application of risk
management to medical devices
(ISO 14971:2000)
•ENISO14971:2000/A1:2003
•EN60601-1:1990
Medical electrical equipment -
Part 1: General requirements for safety
Amendment A1:1993 to EN 60601-1:1990
Amendment A2:1995 to EN 60601-1:1990
Amendment A13:1996 to EN
60601-1:1990
•EN60601-1-2:2001
Medical electrical equipment -
Part 1-2: General requirements for safety
- Collateral standard: Electromagnetic
compatibility - Requirements and tests
•EN60601-1-4:1996
Medical electrical equipment -
Part 1-4: General requirements for safety
- Collateral standard: Programmable
electrical medical systems
Amendment A1:1999 to EN
60601-1-4:1996
•EN60601-1-6:2004
Medical electrical equipment -
Part 1-6: General requirements for safety -
Collateral standard: Usability
•EN60601-2-41:2000
Medical electrical equipment -
Part 2-41: Particular requirements for
the safety of surgical luminaires and
luminaires for diagnosis
Ce produit a fait l’objet de vérifications
conformément aux normes complémentaires
suivantes : CAN/CSA-C22.2 No. 601.1-M90
(R2005) (comprend les différences natio-
nales pour le Canada), EN 60601-1:1990 +
A1:1993 + A2:1995 + A13:1996, UL 60601-1,
1ère édition, 2006-04-26 (comprend les dif-
férences nationales pour les Etats-Unis).
Marquage CE
La conformité aux exigences de la Direc-
tive 93/42/CEE du 14 juin 1993 relative aux
dispositifs médicaux a été évaluée selon
l'Annexe VII de la Directive. La gamme
d’éclairage opératoire PRISMALIX appartient
à la Classe I selon l'Annexe IX de la Directive
93/42/CEE.
Qualitycompliance
Certification of MAQUET SA quality
system
LNE/G-MED certies that the quality system
created by Maquet SA for the design,
manufacturing, marketing, installation and
customer servicing of its surgical lights meets
the requirements of the following international
standards:
- ISO 9001:2000
- ISO 13485:2004
PRISMALIX is designed to fullll the following
applicable standards:
• EN ISO 14971:2000
Medical devices - Application of risk
management to medical devices
(ISO 14971:2000)
•ENISO14971:2000/A1:2003
•EN60601-1:1990
Medical electrical equipment -
Part 1: General requirements for safety
Amendment A1:1993 to EN 60601-1:1990
Amendment A2:1995 to EN 60601-1:1990
Amendment A13:1996 to EN
60601-1:1990
•EN60601-1-2:2001
Medical electrical equipment -
Part 1-2: General requirements for safety
- Collateral standard: Electromagnetic
compatibility - Requirements and tests
•EN60601-1-4:1996
Medical electrical equipment -
Part 1-4: General requirements for safety
- Collateral standard: Programmable
electrical medical systems
Amendment A1:1999 to EN
60601-1-4:1996
•EN60601-1-6:2004
Medical electrical equipment -
Part 1-6: General requirements for safety -
Collateral standard: Usability
•EN60601-2-41:2000
Medical electrical equipment -
Part 2-41: Particular requirements for
the safety of surgical luminaires and
luminaires for diagnosis
This product was investigated according
to the following additional standards:
CAN/CSA-C22.2 No. 601.1-M90 (R2005)
(includes National Differences for Canada),
EN 60601-1:1990 + A1:1993 + A2:1995
+ A13:1996, UL 60601-1, 1st Edition,
2006-04-26 (includes National Differences
for USA).
CE Marking
Compliance with the requirements ofDirective
93/42/EEC dated of June 14th 1993, relating
to medical devices has been assessed in
accordance with Annex VII of this Directive.
This PRISMALIX Surgical lights range is a
class I device in accordance with Annex IX
of Directive 93/42/EEC.
Afin de garantir toutes les
qualités de nos produits, il
est nécessaire de prévenir
MAQUET SA en cas de chan-
gement d'environnement ou
d'utilisation.
In order to guarantee the quality
of our products, it is necessary
to contact MAQUET SA in
case of use or environment
change.