4
Rev. 1 del 24.10.2017
Table of contents
TECHNICAL FEATURES ..................................................................................................................................... 6
Device ................................................................................................................................................................ 6
Use conditions .................................................................................................................................................. 6
Technical features of the currents.............................................................................................................. 6
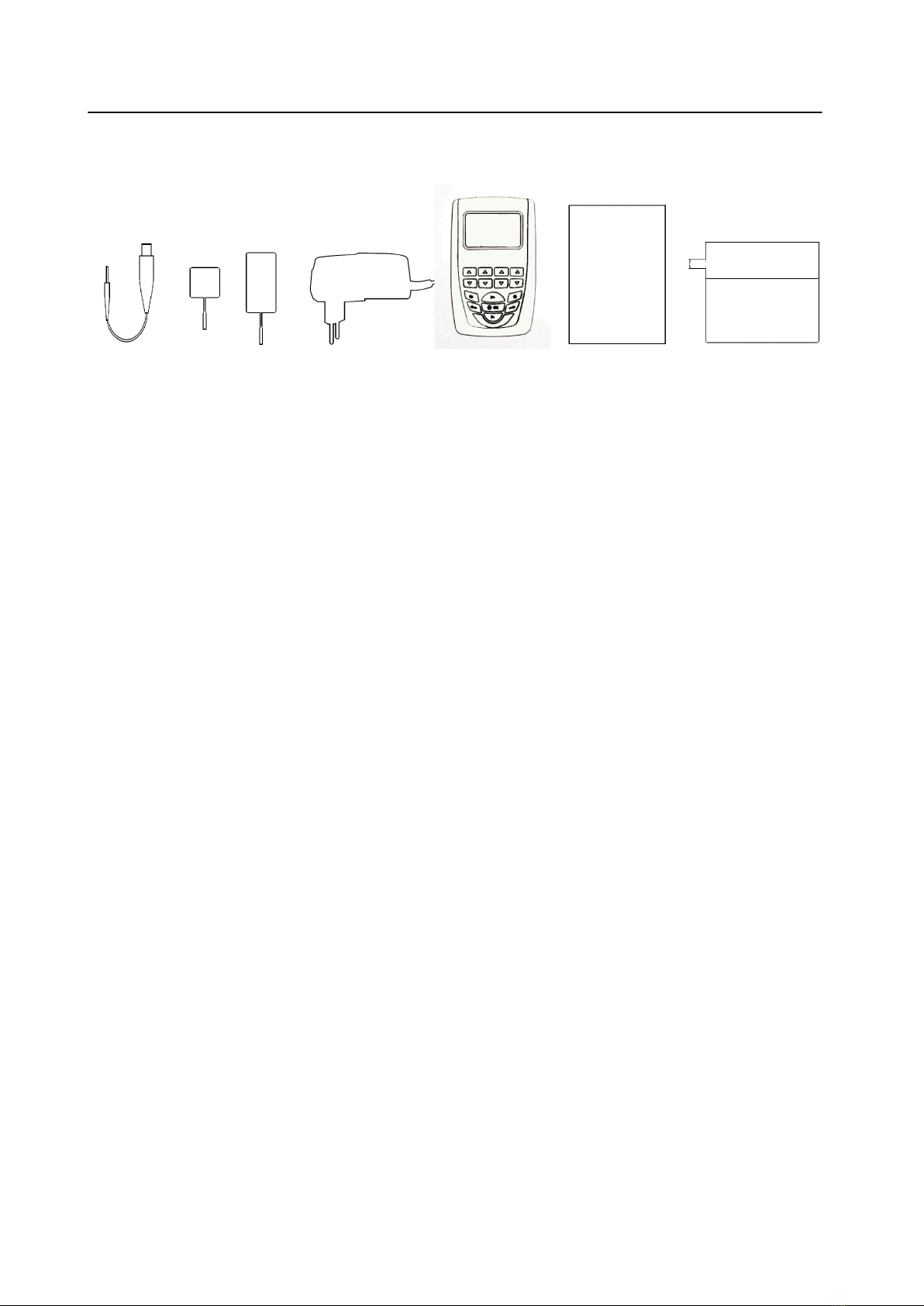
EQUIPMENT ........................................................................................................................................... 8
INTENDED USE ....................................................................................................................................... 9
CONNECTIONS ................................................................................................................................... 10
How to connect the cables ....................................................................................................................... 10
Electrode application .................................................................................................................................. 10
Battery: how to charge the batteries...................................................................................................... 11
LABELLING AND SYMBOLS ........................................................................................................................... 12
Device .............................................................................................................................................................. 13
PANEL AND KEYBOARD................................................................................................................................ 15
Display and interface................................................................................................................................... 16
ALARMS ............................................................................................................................................................ 16
Compliance.................................................................................................................................................... 16
WARNINGS AND CONTRAINDICATIONS.................................................................................................. 17
Mandatory behavior .................................................................................................................................... 17
Warnings before use..................................................................................................................................... 17
Warnings during the use.............................................................................................................................. 18
Side effects...................................................................................................................................................... 19
Contraindications.......................................................................................................................................... 19
MAINTENANCE AND CLEANING ................................................................................................................ 20
Device .............................................................................................................................................................. 20
Battery .............................................................................................................................................................. 20
Accessories ..................................................................................................................................................... 21
Disposal of the device ................................................................................................................................. 21