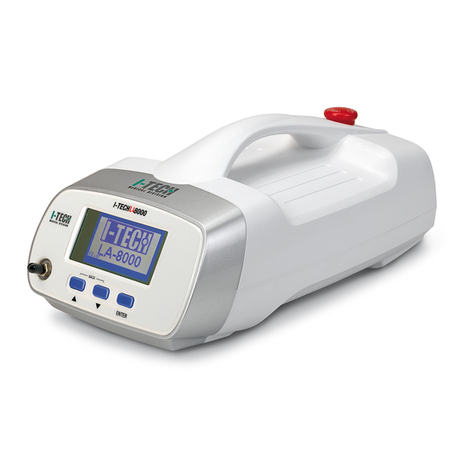
I-Tech MAG700 User manual
Other I-Tech Medical Equipment manuals

I-Tech
I-Tech Mio-Sonic User manual

I-Tech
I-Tech MAG 1000 User manual
I-Tech
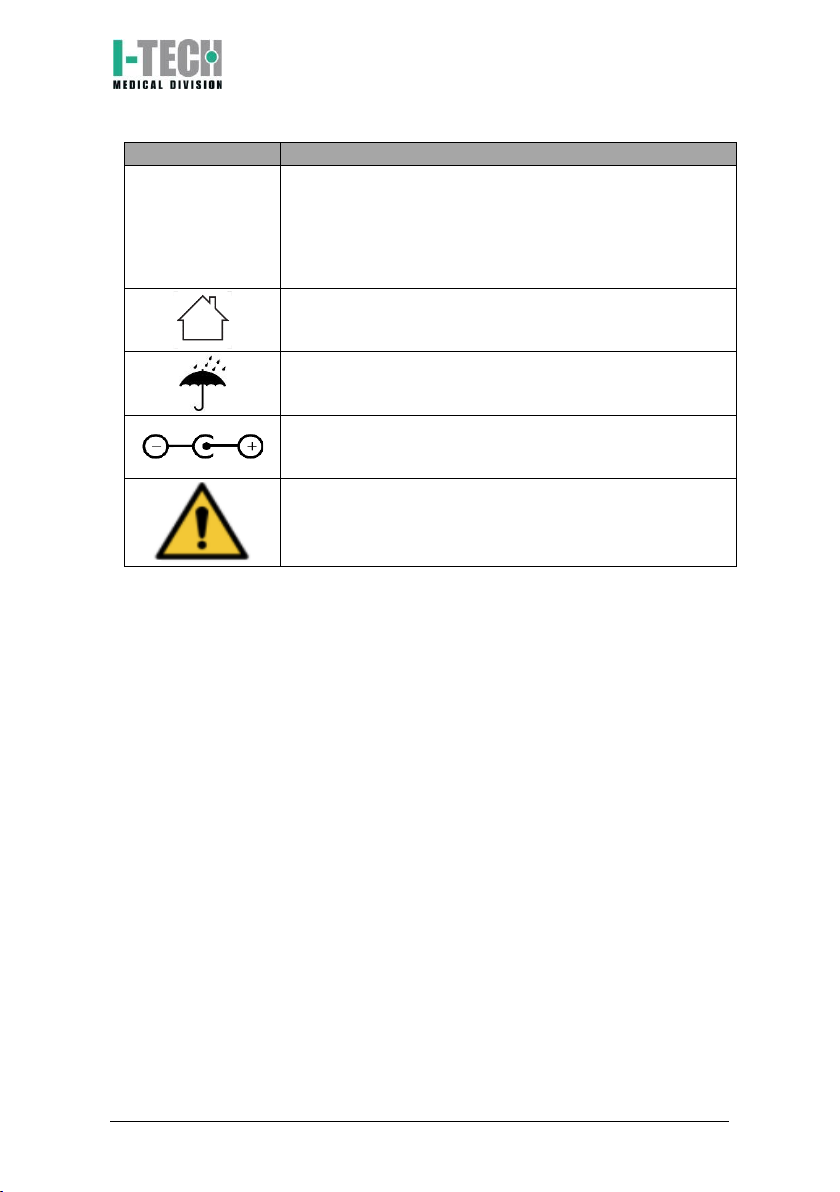
I-Tech UT2 User manual

I-Tech
I-Tech MIO-CARE User manual

I-Tech
I-Tech MIO-IONOTENS User manual

I-Tech
I-Tech MIO-CARE Tens User manual
I-Tech
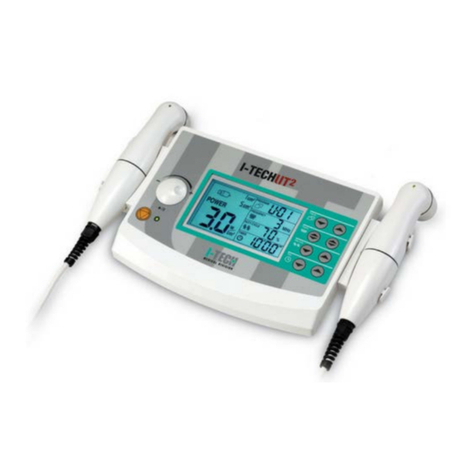
I-Tech UT1 User manual

I-Tech
I-Tech LaMagneto Pro User manual

I-Tech
I-Tech LA500 User manual

I-Tech
I-Tech I-TECH.AR User manual

I-Tech
I-Tech Power-Q1000 Premium User manual

I-Tech
I-Tech I-Press User manual

I-Tech
I-Tech MIO-IONOTENS User manual

I-Tech
I-Tech WonJin Power Q-6000 User manual

I-Tech
I-Tech TAP2000 User manual

I-Tech
I-Tech Mio-Sonic User manual
I-Tech
I-Tech FOX-350 User manual

I-Tech
I-Tech Power Q-6000 Plus User manual

I-Tech
I-Tech PHYSIO 4 User manual

I-Tech
I-Tech MAG2000 User manual