WatchPAT™300 System 2 Operation Manual
11. The tracings and calculations provided by the WP300 system are intended as tools
for the competent diagnostician. They are explicitly not to be regarded as a sole
incontrovertible basis for clinical diagnosis.
12. In the event that the system does not operate properly, or if it fails to respond to the
controls in the manner described in this Manual, the operator should refer to the
Troubleshooting section. If necessary, contact our service office to report the
incident, and to receive further instructions.
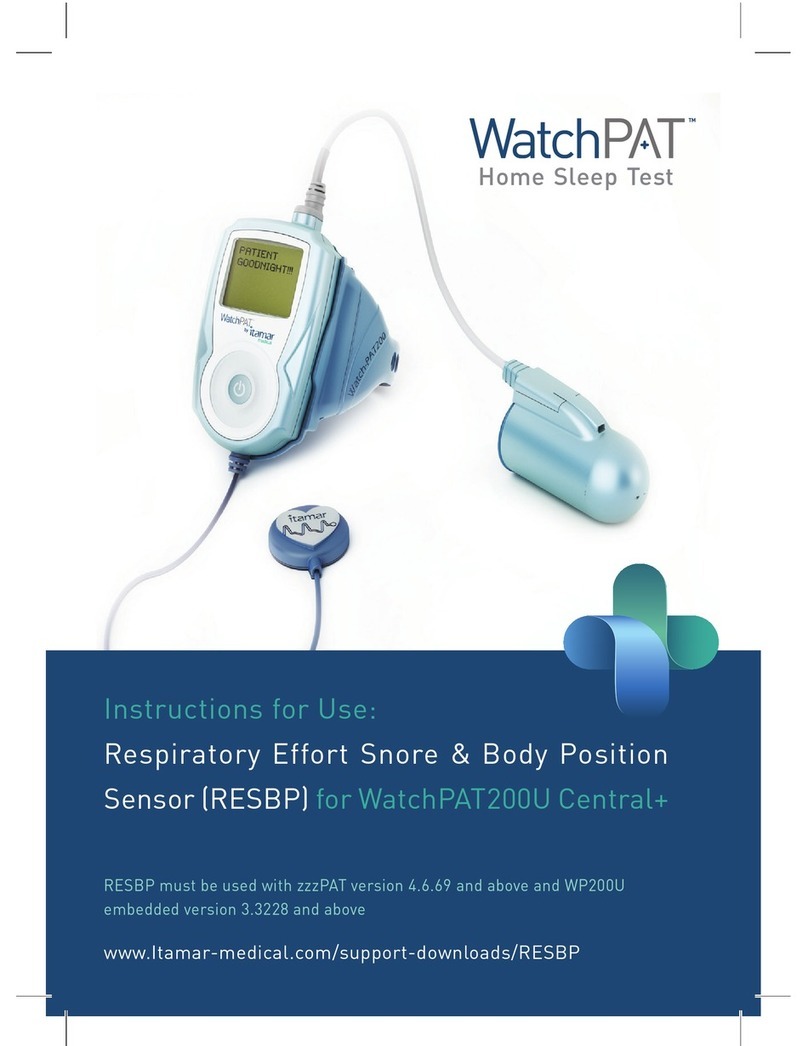
13. The “Step-by-Step Reference Guide”for the patient should be carefully followed
when attaching the unit to the patient.
14. The WP300 is not indicated for patient with injuries, deformities or abnormalities
that may prevent proper application of the WP300 device.
15. The WP300 is not indicated for children less than 12 years old.
1.3 Exclusion Criteria
The WatchPAT™300 should not be used in the following cases:
1. Use of one of the following medications: alpha blockers, short acting nitrates (less
than 3 hours before the study).
2. Permanent pacemaker: atrial pacing or VVI without sinus rhythm.
3. Sustained* non-sinus cardiac arrhythmias.
* In cases of patient having accumulative time of regular R-R intervals of less than
1.5 hours, the WatchPAT™300 system will not have sufficient valid PAT®signal as
required to generate a sleep report.
4. The WatchPAT™300 is not indicated for children who weigh less than 65 lbs.
1.4 Additional Precautions specific to pediatric use
The WatchPAT™300 is indicated for use in patients 12 years and above.
The following Precautions and Notes are referring to pediatric aged 12-17 years.
Precautions:
1. Pediatric patients with severe comorbidities such as Down syndrome,
neuromuscular disease, underlying lung disease or obesity hypoventilation should
be considered for sleep study in a laboratory polysomnograph (PSG) rather than a
home sleep testing (HST).
2. It is recommended that the physician makes sure that the patient and his/her
guardian are aware that the use of specific drugs and other substances used to treat
ADHD, antidepressants, corticosteroids, anticonvulsants, use of caffeine, nicotine,
alcohol and other stimulants might interfere with sleep and affect the sleep study's
conditions.
Notes:
1. PAT Respiratory Disturbance Index (PRDI) is indicated for patients 17 years of age
or greater
2. The snoring and body position safety and effectiveness was not validated on
pediatric patients