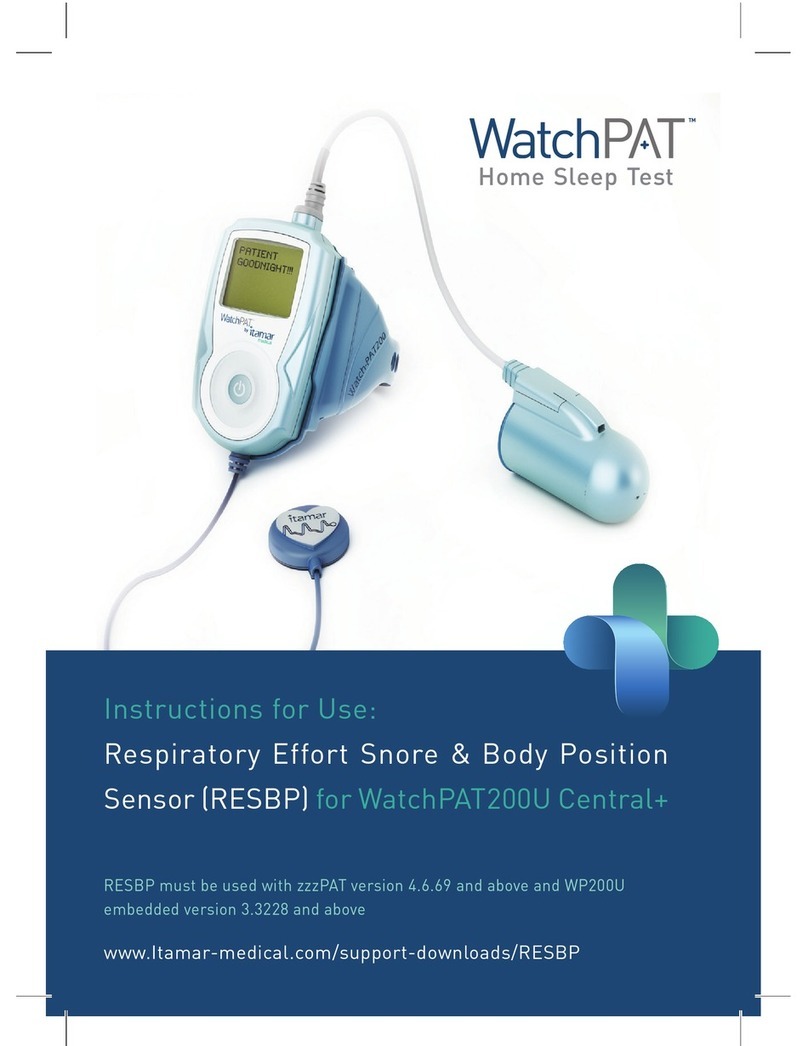
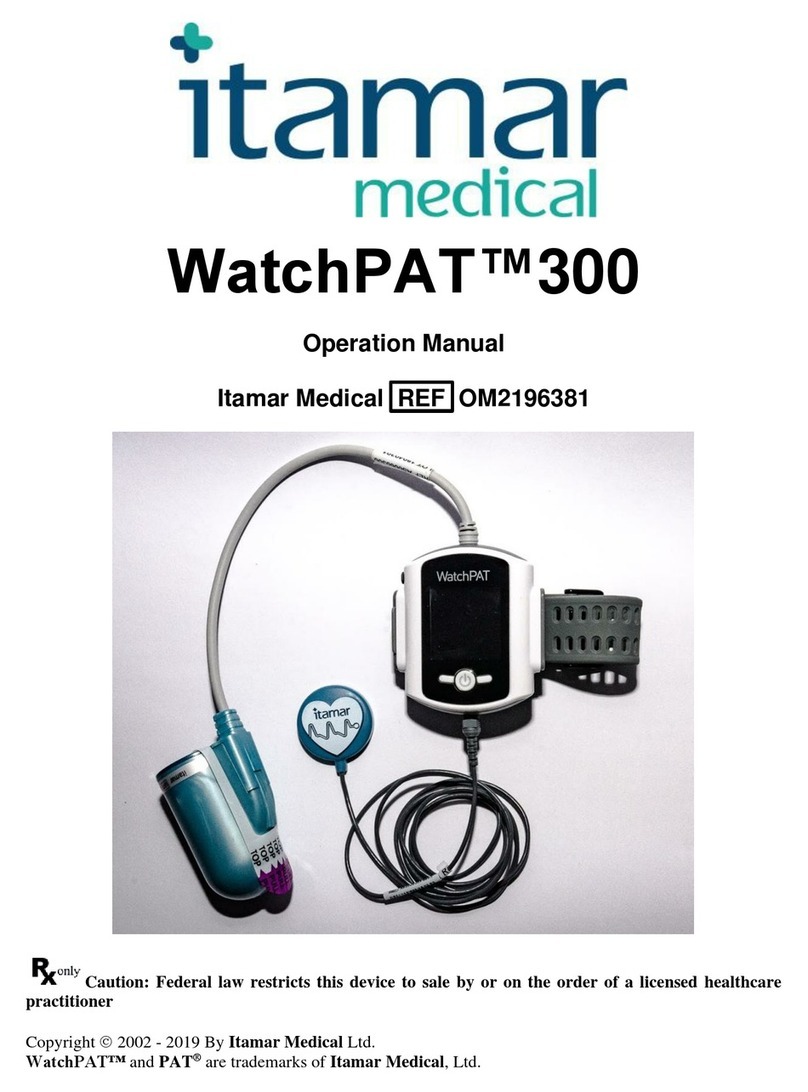
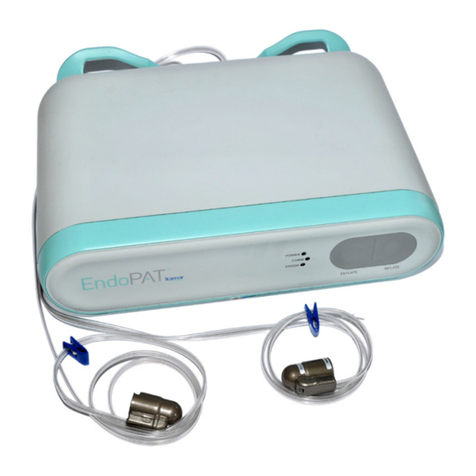
Itamar Medical Ltd.
EndoPATTM2000 Device 1 Operation Manual
1General Information
This manual is part of the EndoPATTM2000 system.
1.1 Intended Use of the EndoPATTM2000 Device
The EndoPATTM2000 device is a non-invasive device, intended for use as a diagnostic aid
in the detection of coronary artery Endothelial Dysfunction (positive or negative) using a
reactive hyperemia procedure.
The Endo PATTM2000 Device has been shown to be predictive of coronary artery
Endothelial Dysfunction in the following patient population: patients with signs or
symptoms of ischemic heart disease, who are indicated for coronary artery angiography, but
who lack angiographic evidence of obstructive coronary artery disease. The device is
intended to be used in a hospital or clinic environment by competent health professionals
The Endo PATTM2000 device is not intended for use as a screening test in the general
patient population. It is intended to supplement, not substitute, the physician’s decision-
making process. It should be used in conjunction with knowledge of the patient’s history
and other clinical findings.
1.2 Performance and clinical study information
The following sensitivity and specificity data were revealed from a clinical study that was
performed at the Mayo Clinic Rochester, MN and that had been designed to evaluate the
safety and effectiveness of the EndoPATTM2000 device as an aiding tool in the diagnosis of
coronary artery Endothelial Dysfunction versus a Gold Standard for coronary Endothelial
Dysfunction evaluation, the Intra-coronary Acetylcholine (Ach) Challenge method:
All subjects: Sensitivity = 82% (45/55), 95% lower confidence bound = 71%
Specificity = 77% (30/39), 95% lower confidence bound = 63%
________________________________________________________________
Females: Sensitivity = 91% (30/33), 95% lower confidence bound = 78%
Specificity = 74% (17/23), 95% lower confidence bound = 55%
Males: Sensitivity = 68% (15/22), 95% lower confidence bound = 48%
Specificity = 81% (13/16), 95% lower confidence bound = 58%
The Gold Standard for Endothelial Dysfunction evaluation, the Intra-coronary
Acetylcholine (Ach) Challenge method, is routinely performed at the Mayo Clinic.
According to the Intra-coronary Acetylcholine (Ach) Challenge method, a catheter is
positioned in the origin of the left main coronary artery and Ach is infused with incremental
concentration followed by coronary angiogram. The coronary artery diameter is measured
in the segment 5mm distal to the tip of a Doppler wire using a computer-based image