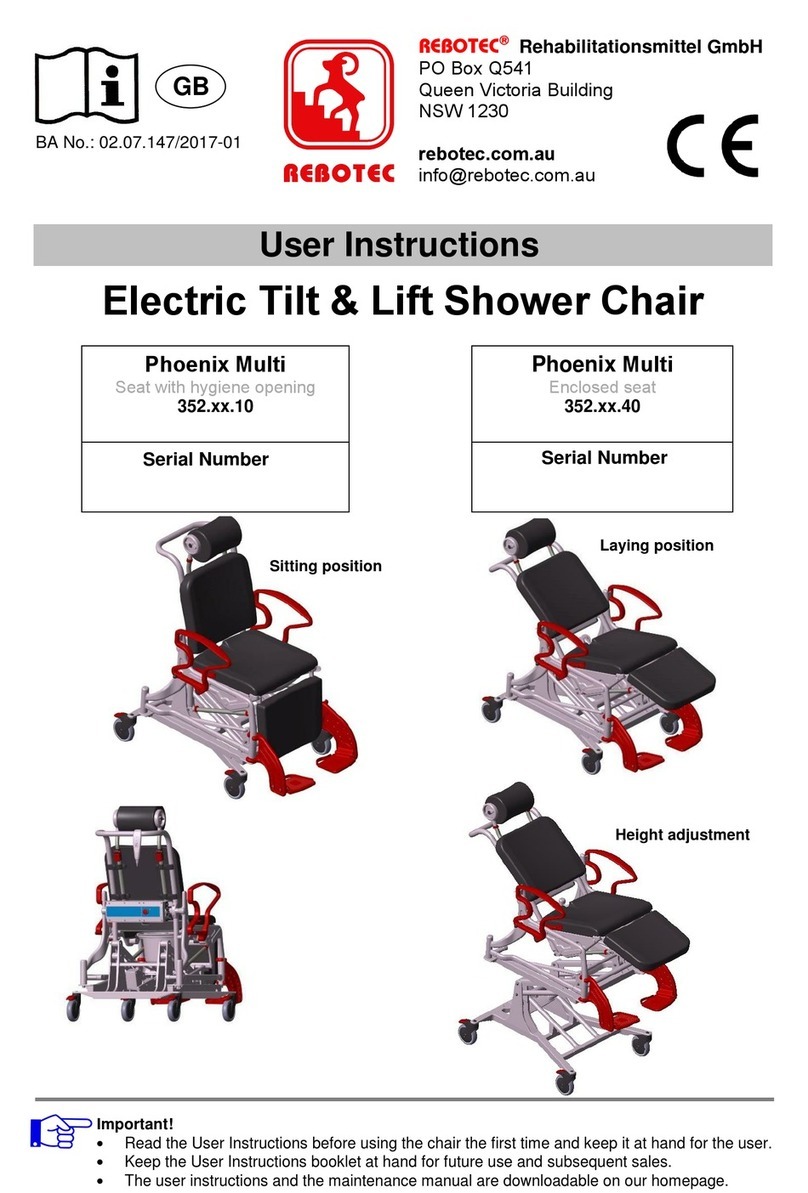
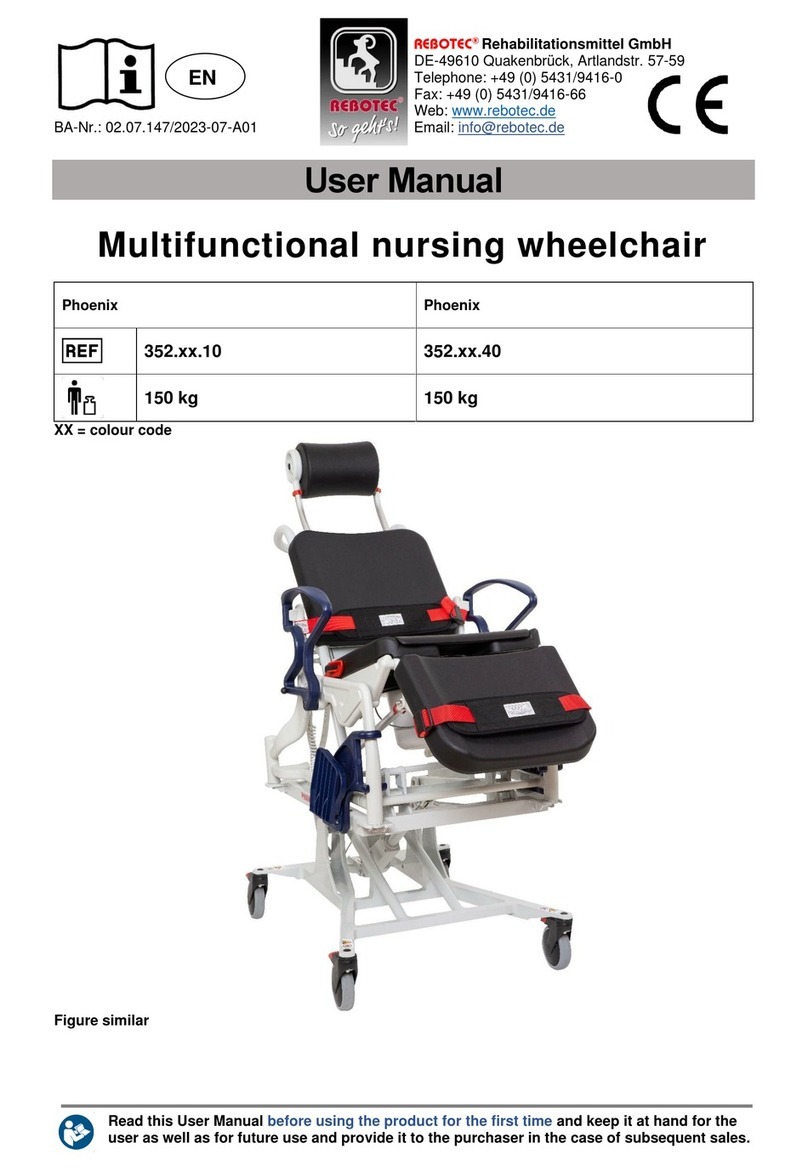
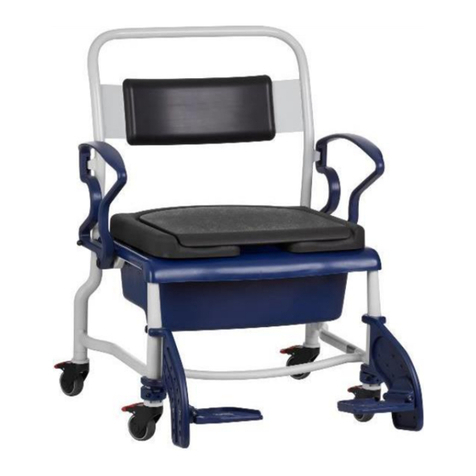
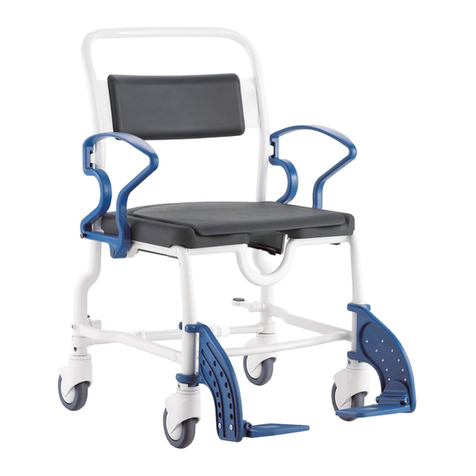
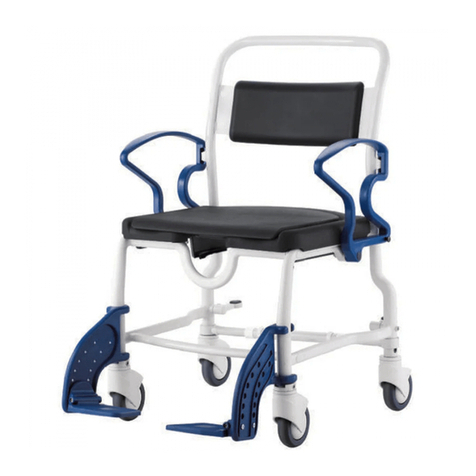
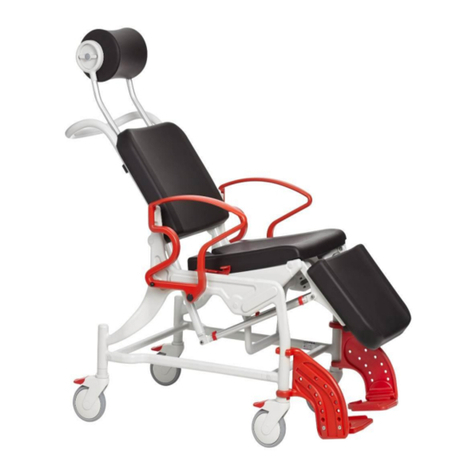
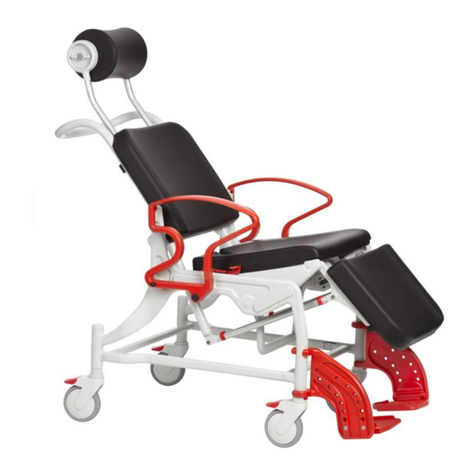
REBOTEC / WA-No.: 02.11.151/2018-09 GB
Maintenance of chairs
1.1 Maintenance / inspection requirements
In this maintenance instructions pamphlet you’ll
find information and advisories on the safe and
professional maintenance and/or repair work on
the Multifunctional wheel chairs.
The products are Class I medical products as to
the 93/42/EEC and the German MPG/Medical
Products Act.
As to the requirements of the German MPBetreibV
Medical Products Ordinance, the products must be
maintained and the hygiene requirements must be
adhered to. Check your local requirements.
The product’s lifetime is in large part affected by
how it is used. The frequency of use and manner
of use reduces the lifetime as does improper
handling and inadequate maintenance.
In order to ensure safe use, a visual inspection and
function check must be done at least once per
year. Shorter time intervals between maintenance
may be necessary when the frequency of use or
the condition of the product exists due to safety
reasons.
The operator has to carry out the maintenance on
his own or must instruct a competent person
(specialized supplier, medical supply store or a
medical-maintenance-service) if he has not the
needed expertise.
The products with electric or hydraulic components
must be checked by a person with the
corresponding expertise.
Inspection results must be documented and be
confirmed by a signature. The product must be
provided with an inspection sticker that states the
next inspection. The product operator is
responsible for assuring regularly-conducted
inspections.
1.2 Maintenance scope
The product’s inspection extends from the general
condition to the individual components, function
and safety.
Necessary repair wroks must be done without
delay. If an immediate repair work is not
possible, then it is in the patient’s interest and
health to put the product out of service.
1.3 Maintenance advisories
When making repairs, use only original spare parts
from REBOTEC.
Do not undertake any modifications or conversions
to the product on your own. These will adversely
impact the product’s safety and function.
REBOTEC accepts no liability in such cases.
When performing maintenance and repairs, please
use the product’s User Instructions booklet as an
aid and follow the instructions concerning proper
use, re-use, lifetime, guarantee, care and
maintenance.
After completing maintenance or repair work on
the product, follow the requirements for hygiene for
re-use.
Important hints!
Do not open the housing of the electric
components.
Check the hydraulic unit for tightness.
In case of damage replace the whole
hydraulic unit.
1.4 Maintenance reports and overview
In the appendix you will find a form for
documenting maintenance. You can make copies
of the form. This allows you to keep an overview of
the product’s maintenance status.
Maintenance protocol (single report)
It is a form for proof of a single
maintenance session.
Maintenance overview (total report)
It is a form for proof of executed
maintenance sessions.
1.5 Disposal
For disposing of the product, follow all the
advisories in the product’s User Instructions
booklet. Disposal must be done at a recycling
company. Contact your local recycling company for
more information.
1.6 Transport and storage
When transporting or storing the mobility aid for
extended time periods, the emergency OFF
button on the control unit must be pressed in
completely.
Advisory on other documents
At our www.rebotec.de website you can download
the User Instructions booklets, forms and other
information.