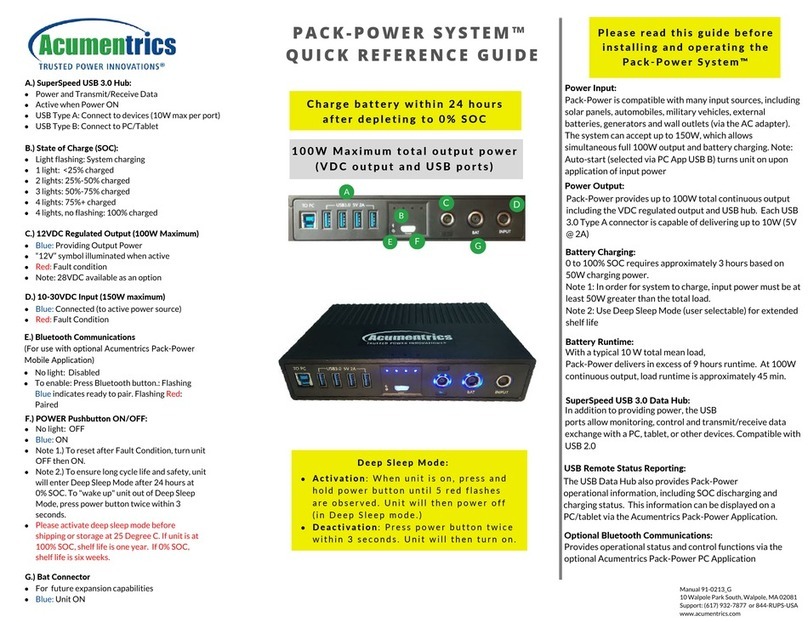
2.1 Complete reliability
The block battery does not suffer from
the sudden death failure associated
with the lead acid battery
(see section 4.1 Plate assembly).
2.2 Long cycle life
The block battery has a long cycle
life even when the charge/discharge
cycle involves 100% depth of
discharge (see section 6.7 Cycling).
2.3 Exceptionally long lifetime
A lifetime in excess of twenty years
is achieved by the Saft Nife block
battery in many applications, and at
elevated temperatures it has a lifetime
unthinkable for other widely available
battery technologies (see section 6.8
Effect of temperature on lifetime).
2.4 Low maintenance
With its generous electrolyte reserve,
the block battery reduces the need
for topping up with water, and can be
left in remote sites for long periods
without any maintenance
(see section 6.9 Water consumption
and gas evolution).
2.5 Wide operating
temperature range
The block battery has an electrolyte
which allows it to have a normal
operating temperature of from -20°C
to +50°C, and accept extreme
temperatures, ranging from as low
as -50°C to up to +60°C
(see section 4.3 Electrolyte).
2.6 Fast recharge
The block battery can be recharged
at currents which allow very fast
recharge times to be achieved
(see 8.3 Charge acceptance).
2.7 Resistance to mechanical
abuse
The block battery is designed to have
the mechanical strength required
to withstand all the harsh treatment
associated with transportation over
difficult terrain
(see section 9.2 Mechanical abuse).
2.8 High resistance to electrical
abuse
The block battery will survive abuse
which would destroy a lead acid
battery, for example overcharging,
deep discharging, and high ripple
currents
(see section 9.1 Electrical abuse).
2.9 Simple installation
The block battery can be used with a
wide range of stationary and mobile
applications as it produces no
corrosive vapors, uses corrosion-free
polypropylene containers and has
a simple bolted connector assembly
system
(see section 10 Installation and
storage).
2.10 Extended storage
When stored in the empty and
discharged state under the
recommended conditions, the block
battery can be stored for many years
(see section 10 Installation and
storage).
2.11 Well-proven pocket plate
construction
Saft has nearly 100 years
of manufacturing and application
experience with respect to the nickel-
cadmium pocket plate product, and
this expertise has been built into the
twenty-plus years design life of the
block battery product
(see section 4 Construction features
of the block battery).
2.12 Environmentally safe
More than 99% of the nickel-
cadmium block battery can be
recycled, and Saft operates a
dedicated recycling center to recover
the nickel, cadmium, steel and plastic
used in the battery.
2.13 Low life-cycle cost
When all the factors of lifetime, low
maintenance requirements, simple
installation and storage and
resistance to failure are taken into
account, the Saft Nife block battery
becomes the most cost effective
solution for many professional
applications.
4
2. Benefits of the block battery
BlockBat 3/11/98 10:11 Page 7
Home BackHome BackHome Back