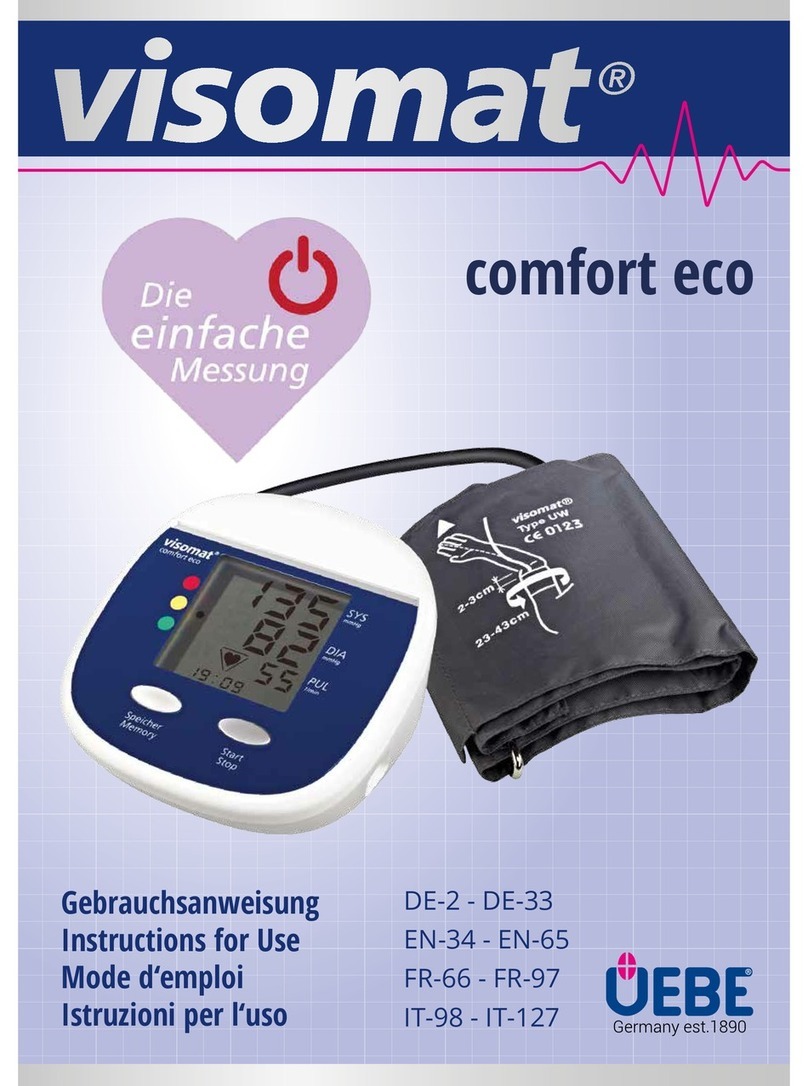
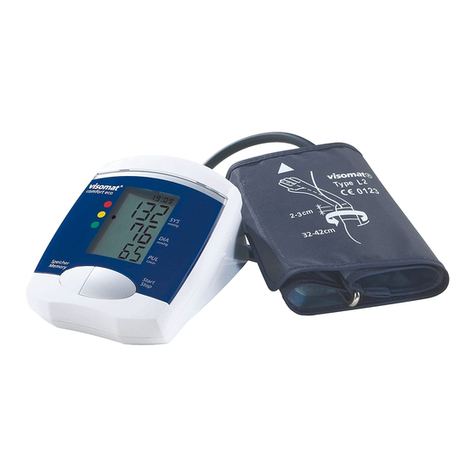
Failure and error messages1.
Failure en-
countered
Possible cause Corrective action
Display LO Pulse below 40, unit can only
display pulse between 40 and 200.
Interrupted pulse tones? Listen to
the peep signal attentively, possibly
consult doctor.
Irregular heartbeat, disruptive
movements, shaking, wobbling
(objects), breathing in deeply, etc.
Repeat measurement after
3-5 minutes rest. Possible factors
(depending on the severity of the
arrhythmia) affecting the measure-
ment results must be discussed
with your doctor!
Measured
values too
high
Was the necessary rest observed
prior to measurement?
Repeat measurement after a break
of approx. 3-5 mins.
- Do not move arm
- Do not talk
Has the proper cuff been selected? Cuffs that are too small result in
excessive blood pressure values.
Measure your arm circumference in
the middle of the upper arm.
Measurement
values too
low
Upper arm cuff too big. Please use standard cuff.
Check arm circumference.
Unusual
measured
values
Movement or talking during
measurement, resting time not
observed, feet possibly crossed,
smoking or coffee cunsumption.
Check conditions and repeat
measurement. Observe instructions
for use P. EN-36.
No display
after unit is
switched on
Batteries inserted incorrectly? Check position of batteries.
Batteries flat? Change batteries.
Battery compartment dirty? Clean battery compartment.
Measurement
interrupted
Batteries flat? Change batteries.
Failure en-
countered
Possible cause Corrective action
Display Err 1 The systolic pressure has been
determined, but the cuff pressure
fell below 20 mmHg afterwards.
This situation occurs if the hose
has been disconnected after having
measured the systolic blood pres-
sure. Further possible causes: No
pulsations could be detected.
Large leakage during measure-
ment. If plug-and-socket connected
ok, send in.
Display Err 2 Unnatural pressure pulse influence
the measurement result. Reason:
The arm has been moved during
measurement (artefact).
Relax.
Display Err 3 Inflating the measurement
takes too much time.The cuff is
positioned improperly or the hose
connection is leaky.
If both are not applicable – send
in.
Display Err 5 The unit detected an unacceptable
difference between systolic and
diastolic pressure. Repeat the
measurement und follow the ins-
tructions thoroughly. Please consult
the doctor if the measurement
results continue to be unusual.
Plausibility check of the measure-
ment results. Here, values such
as 160/140 are questioned by
the unit.
Display HI Pulse above 200, unit can only
display pulse between 40 and
200, possible irregular heartbeats,
respiratory arrhythmias.
Irregular heartbeats - repeat
measurement after 5 minutes
of rest.
Respiratory arrhythmias – repeat
measurement after 5 minutes of
rest, no deep breaths, possibly
consult doctor.