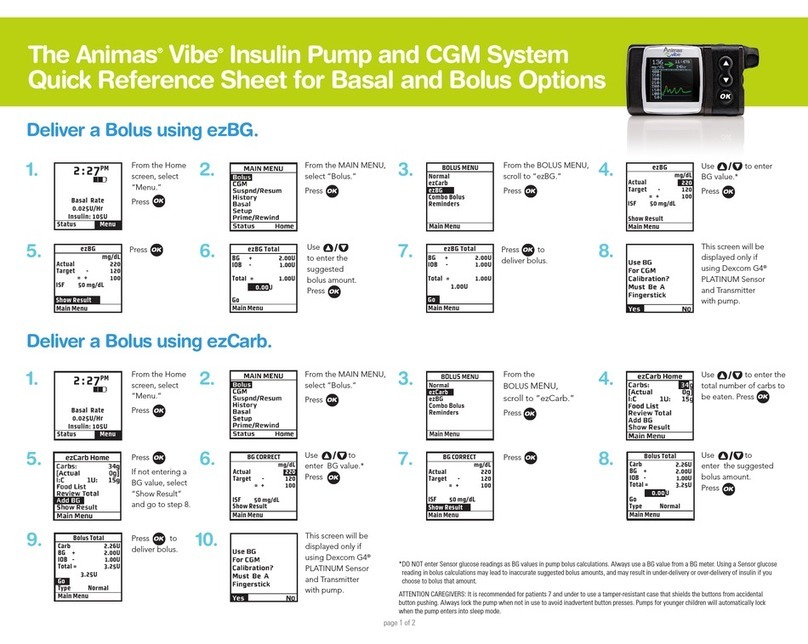
III
BEFORE YOU BEGIN
Warnings, Precautions and Important Safety Information about your
Animas
®
Vibe
™
System
Read the entire Section I and Section II of this Owner’s Booklet before using your Animas®Vibe™
System. If you do not understand something or have any questions, ask your health care team.
Warnings, Precautions, Cautions, Contraindications and other important Safety information about
your Animas®Vibe™System follows below. Be sure to read the information carefully.
Radiology Equipment
Your pump has been designed to operate in the presence of common sources of electrostatic and
electromagnetic interference, such as store security systems. However, like all portable electronic
devices, your pump should not be exposed to very strong electromagnetic fields, such as in Magnetic
Resonance Imaging (MRI), RF welders or magnets used to lift automobiles. Very strong magnetic fields,
such as in an MRI, can re-magnetize the portion of the motor that regulates insulin delivery. If you plan
to undergo an MRI, remove your pump and CGM Transmitter/Sensor beforehand and then keep them
outside the room during the procedure.
WARNINGS:
•Check with your health care provider regarding your individual training needs. Do Not attempt
to connect to your pump before you have been trained on your pump.
•Incorrect use of your pump, failure to follow the instructions in this Owner’s Booklet or
improper/inadequate self-care and troubleshooting techniques can lead to death or serious
injury. If you do not understand something or have questions, ask your health care team
or call Customer Support or your local Animas distributor. In the United Kingdom, call (free
phone) 0800 055 6606. In Ireland, call 1 800 812 715. All other countries, call your local
Animas distributor (see page 187).
•Your pump is designed to deliver insulin reliably, but because your pump uses only rapid-
acting insulin, you will not have long-acting insulin in your body. To avoid the risk of diabetic
ketoacidosis (DKA) or very high BG, you must be prepared to give yourself an injection of
insulin if delivery is interrupted for any reason.
•Your pump is designed and calibrated to deliver U100 insulin. Use of any insulin with lesser or
greater concentration can result in serious injury or death.
•Never start the Prime/Rewind sequence on your pump while the infusion set is connected to
your body. The Prime/Rewind sequence includes steps for rewinding the pump motor, loading
an insulin cartridge and tightening the cartridge cap, and priming the infusion set tubing.
Failure to disconnect your infusion set from your body before performing these steps can
result in over delivery of insulin, and possible injury or death. If your pump sustains internal
damage, the amount of unintended insulin delivery could be significant. This could result in
serious injury or death from hypoglycæmia.
• You must take a fingerstick test with your BG meter and use that BG value to make any insulin
or treatment decisions. Insulin dosing decisions should not be based solely on results from
the CGM.
•Your pump and pump accessories include small component pieces that could pose a choking
hazard for small children.
41012850A_OB_Unity_OUS_EN_mmolL.indd 3 3/2/11 2:09 PM