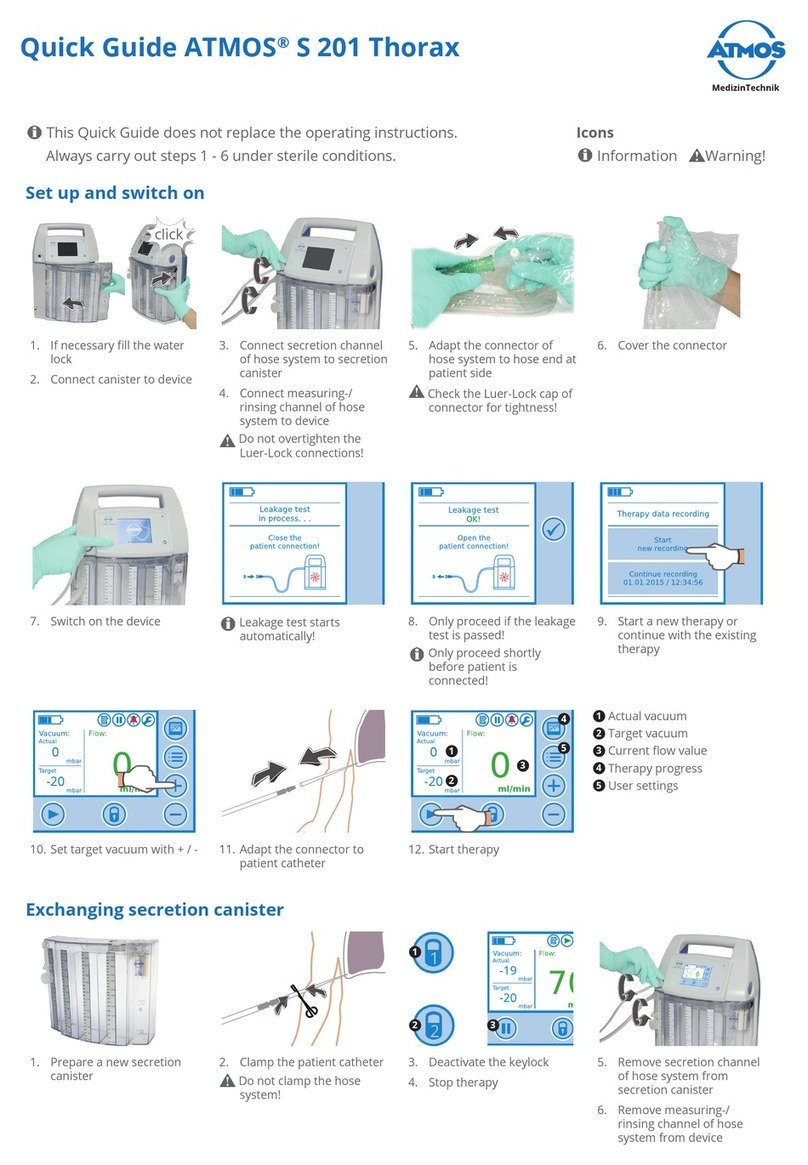
Atmos C 451 User manual

ATMOS C 451
Surgical Suction Unit
340.0300.B USA
2013-08 Index: 17
Operating Instructions
English

2
ATMOS
MedizinTechnik GmbH & Co. KG
Ludwig-Kegel-Str. 16
79853 Lenzkirch
Deutschland / Germany
Tel. + 49 (0) 76 53 / 689-0
Fax: + 49 (0) 76 53 / 689-190
+ 49 (0) 76 53 / 689-393 (Service Center)
www.atmosmed.de
5.0 Options .........................................................11-13
5.1 Foot switch..........................................................11
5.2 System trolley .....................................................11
5.2.1 Securing the unit.................................................12
5.2.2 Travelling with the trolley ....................................12
5.3 Tray in foot of system trolley ...............................13
5.4 Potential equalisation..........................................13
5.5 Use of suction unit with disposable systems.......13
5.6 Battery Backup System.......................................13
6.0 Cleaning....................................................... 14-15
6.1 General information on cleaning
and disinfection...................................................14
6.2 Cleaning and sterilizing the unit surface .............15
6.3 Recommended disinfectants for instruments......15
6.4 Recommended disinfections for surfaces...........15
7.0 Maintenance ......................................................15
7.1 Change fuse........................................................16
8.0 Trouble-shooting...............................................16
9.0 Accessories, consumables and
spare parts................................................... 18-18
10.0 Technicalspecications..................................19
11.0 Checking / Reprocessing / Disposal...............20
11.1 Checking ATMOS suction devices......................20
11.2 Reprocessing......................................................20
11.3 Disposal ..............................................................20
12.0 Notes on EMC.............................................. 21-23
General Standard Terms and Conditions
Content
Page
1.0 Introduction..................................................... 3-5
1.1 Notes on operating instructions ............................3
1.2 Intended use .........................................................3
1.3 Function ................................................................3
1.4 Explanation of pictures and symbols ....................4
1.5 Scope of supply ....................................................5
1.6 Transport and storage...........................................5
2.0 For your safety ...................................................6
3.0 Setting up ........................................................ 7-8
3.1 Operating elements...............................................7
3.2 Connection area in unit base ................................8
4.0 Operating....................................................... 9-10
4.1 On/off switch .........................................................9
4.12 Set vacuum...........................................................9
4.3 Suction................................................................10

3
1.2 Intended use
The Surgical Suction Unit ATMOS C 451 is a compact suction
unit for medical application. It is especially intended for aspira-
tionandcollectionofsecretions,bodyuidsandtissue.
Itsmaineldsofapplicationare:
– in the OPD, in the OR: for sucking off and collecting e.g.
drain pockets, abscesses, body and rinsing solutions
and during lipectomy;
– in endoscopy: e.g. to aspirate secretions or rinsing so-
lutionsaswellasforoperativexation;
– in gynaecology: for suction curettage;
– In ENT applications: to aspirate secretions, rinsing so-
lutions, cerumen or to extract foreign matters;
– in the ward, recovery ward and ICU: : for the spon-ta-
neousaspirationofbodyuidsandforeignmatters,e.g.
from the respiratory tract.
Contraindications for Use:
– Exposed arteries, veins or organs, Untreated
osteomyelitis, Necrotic tissue, Malignancies, and
Fistulas.
– for smoke evacuation in connection with
HF-electrosurgery or laser surgery.
1.3 Function
The ATMOS C 451 is a line-power operated surgical suction
unit, centering around a silent diaphragm-type pump which
generates a vacuum inside a collection jar. Using a vacuum
regulator and the vacuum-gauge, the target vacuum and
thustheair-owratecanbepreciselyadjusted.
A system trolley is available for mobile use.
1.0 Introduction
1.1 Notes on operating instructions
These operating instructions contain important notes on how to operate the ATMOS C 451
safely, correctly and effectively. Reading this booklet helps avoid risks, and also to reduce repair
costs
and down-time. That increases, amongst other things, the reliability and service-life of the device.
These operating instructions serve not only for new operating personnel to be instructed in its use,
but also for use as a reference manual. Any reprint - even in extracts - only after written permission
from ATMOS.
These operating instructions must always be kept available near the device.
Care and safety inspections in conjunction with professional execution provide for operational safety
and readiness for use of your ATMOS C 451 and are therefore a must besides regular cleaning.
Repair work and safety inspections may be carried out only by expert personnel authorised by
ATMOS. By applying only original spare parts you will have the guarantee that operational safety,
readiness for work and the value of your ATMOS C 451 will be preserved.
Warning:
– Do not use in non-medical applications;
– Do not use for emergency medical aid.
– Do not use in the presence of combustible or
explosiveuidsorgases.
– Do not use for drainage in the low vacuum range
(e.g. thorax drainage).
Caution: Federal law restricts this device to sale by or
on the order of a physician.
●The product ATMOS C 451 bears CE marking CE 0124
according to the EC Directive of the
council for medical products 93/42/EEC and meets the
basic requirements of appedix I of this
directive.
●The quality management system applied at ATMOS
hasbeencertiedaccordingtointernational
standards EN ISO 9001 and EN ISO 13485.
●Prior to start-up please peruse chapter 2.0
„For your safety“, in order to be prepared for any
possible dangerous situations.
●The product ATMOS C 451 bears CE marking CE 0124 according to the EC Directive of the
council for medical products 93/42/EEC and meets the basic requirements of appedix I of this
directive.
●ThequalitymanagementsystemappliedatATMOShasbeencertiedaccordingtointernational
standards EN ISO 9001 and EN ISO 13485.
●Prior to start-up please peruse chapter 2.0 „For your safety“, in order to be prepared for any
possible dangerous situations.

4
1.0 Introduction
!
■
●
→
click
click
Important information
Keys on the control panel / symbols at the ATMOS C 451
REF
SN
~
Foot switch
IPX1
Observe operating instructions
1.4 Explanation of pictures and symbols
Short cuts / symbols contained in this manual
Please press where
dot indicates
Subnumeration
Numeration
General information
Follow the arrows
whilst proceeding,
sequence
Replace
Check
Please read,
important information
Move, plug ... in this
direction
Engage, check correct
t
Turn, shift ... in this
direction
Warning, especial diligent
notice
Pictures contained in this manual
Off (feed-in, power
connection)
On (feed-in, power
connection)
Alternating current
Application part type BF
Fuse
Potential equalization
The CE sign shows that this
product meets the appropriate
requirements of the
EC Directives
Serial number
Order number
Creation date
Protection class II
Protection against penetration of
damaging humidity (drop water)

5
1.0 Introduction
Basic device
Operating
instructions
Mains cable Trolley
Optional:
1.5 Scope of supply
●Prior to dispatch, this ATMOS device was subjected to an extensive functional test and has been carefully packed.
Nevertheless, please compare the contents of the shipment on completeness immediately upon receipt (see delivery note).
In addition to the basic device, the scope of delivery comprises the following parts:
1.6 Transport and storage
● Thetransportofthedevicemaybeeffectedonlyinadispatch
cartonupholsteredandofferingsufcientprotection.
● Pleasedocumentandreportdamagesintransitimmediately.
For complaints or return deliveries, please use the enclosed
form QD 434.
●The unit must be allowed to stand for up to six hours at room
temperaturepriortostartingupforthersttimefollowing
transport at temperatures below freezing. The unit may not
be operated if it has not acclimatised as this might damage its
diaphragms.
●Ambient conditions:
Transport/Storage: -22...+122°F;
5...90 % humidity
non-condensing
at air pressure 700...1060 hPa
Operation: +50...+95°F;
20...80 % humidity
non-condensing
at air pressure 700...1060 hPa
System
trolley

6
!
For your safety
The ATMOS C 451 fully complies with the electromagnetic
immunity requirements of standard IEC 60601-1-2 / EN
60601-1-2 "Electromagnetic compatibility - Medical Elec-
trical Equipment".
Warranty period for this unit: 2 years. This period is
unaffected by any repair or maintenance carried out under
the terms of the warranty. Please also pay attention to our
enclosed General Standard Terms and Conditions.
The warranty will be rendered invalid in case of damages
caused due to the utilization of accessories or consumables
which are not approved by ATMOS for use with this unit.
ATMOS is not liable for personal injury and damage to
property if
• nooriginalATMOSpartsarebeingused,
• theadviceforuseintheseoperatinginstructionsisnot
being observed,
• assembly, new settings, alterations, extensions and
repairs have been carried out by personnel not
authorised by ATMOS.
This operation manual corresponds with the construction
of the unit and with the current status of safety-related
standards at the time of printing. Proprietary rights are exi-
sting for all described circuits, processes, names, software
programs and units.
The design of the ATMOS C 451 fulllstherequirements
of IEC 60601-1/EN 60601-1 and of protection class I. The
device must only be connected to a properly installed
socket with non-fused earthed wire.
Before putting the device into operation, visually check
unit, power cable and accessories for signs of damage.
Damaged cables must be replaced immediately. Check
also function of the unit.
The ATMOS C 451 may be used in supervised operation
by qualied personnel only which has been authorised
by ATMOS and which has been trained for operating the
appliance (IEC 60601-1/EN 60601-1).
The ATMOS C 451 may be operated only in rooms used
for medical purposes, but not in areas (zones M and G)
subject to explosion hazards and in oxygen rich envi-
ronments. Explosion harzards may result from the use of
combustible anaesthetic agents, skin cleansing agents or
disinfectants.
The foot switch is suited for operation in above mentioned
areas.
Liquids must not be allowed to enter the device. Should
liquids have penetrated into the device, it must be inspec-
ted (danger of an electric shock) and the pump must be
decontaminated resp. exchanged (infection risk).
After transport at cold temperatures (below the freezing
point), the unit must acclimatize prior to rst use; leave
it unoperated at room temperature for a period of up to 6
hours. If the unit is not acclimatized it must not be operated
as the membranes of the pump might get damaged.
Dispose of the packaging material, observing the appli-
cable waste-control regulations.
Before connecting the device to the power line, check that
the voltage and frequency ratings of the power line are
similar to those indicated on the device.
Never connect the unit to defective power sockets or ex-
tension cables.
Whendisconnectingthedevicefromthepowerline,rst
remove the plug from the wall outlet. Then the power cord
may be disconnected from the device. Never touch the
plug or cord while your hands are wet.
Theambientconditionsspeciedinsection10.0mustbe
strictly observed.
Set up the device so that the operator has a clear, unob-
structed view of and easy access to the front panel. The
device must be placed on a solid, level surface.
This product is not re-sterilisable. Repeated reuse of
components which are marked with a is forbidden.
In case of repeated reuse these components lose their
function and there is a high infection risk.
2.0 For your safety
2

7
Fig. 1.
Fig. 2.
3.0 Setting up
Always set the equipment up on a secure, level surface.
3.1 Operating elements
On/Off switch with pilot lamp
Vacuumgauge
Vacuum controller

8
Fig. 4.
Fig. 5.
Fig. 7.
Fig.6.
3.2 Connection area in unit base
Connect mains cable
Use only mains cables with angled inlet connector for
non-heating appliances!
Check that the voltage and frequency ratings of the power
line are similar to those indicated on the device.
Connect footswitch (optional)
Thread knurled nut onto hose.
Push hose onto connecting nipple.
Tighten knurled nut.
Vacuum connection on base system trolley
Toconnect,presscouplingrmlyintosocketuntillatched
into place (serves for vacuum connection of collection jar
attached to system trolley).
To remove, press the metal interlocking device apart side-
ways and pull the hose out of the socket.
3.0 Setting up

9
Fig. 8.
Fig. 9.
4.1 On/off switch
Press the ”I” symbol to switch the unit on.
Press the ”0” symbol to switch the unit off.
The unit is ready for optional footswitch operation in the
„0“ position (see options).
4.2 Set vacuum
Close the suction hose and set the desired vacuum by
turning the vacuum controller according to the direction of
the arrow.
Do not use force to turn the knob at its limits!
Test the system for leaks if the desired vacuum is not
reached. (Please also see chapter 8.0 Trouble shooting)
4.0 Operation

10
4.3 Suction
Use appropriate suction tubes, catheters, suction tips or
suction instruments, containers and hoses.
Prior to starting suction, containers must be checked for
cracks. Damaged containers may not be used.
Keep an eye on the level of liquid in the collection jar during
suction.
Use only containers with oversuction protection.
4.0 Operation

11
Fig. 10.
Fig 11.
5.0 Options
5.1 Foot switch (REF 340.0060.0)
The foot switch works pneumatically and is therefore sui-
tableforuseinconnection withammableanaesthetics
(class AP).
Connect the foot switch as shown in section 3.2.
Set the unit to ”0” off at the main switch (foot switch
mode).
The unit is switched on by pressing the foot switch and
switched off by pressing it again.
5.2 System trolley
A system trolley, which can be used with a changeover
adapter is available for use in the OT.
Always position the system trolley on a at, sturdy sur-
face.

12
Fig. 12.
Fig. 13.
Fig. 14.
Use the lockable castors if necessary.
5.2.2 Travelling with the trolley
Always push the trolley so that the open fork of the pedestal
faces in the direction of travel.
Push the suction unit using both hands in the handle
area.
Ensure that hoses and cables are secured in place.
Never leave the system trolley on a sloping surface!
5.2.1 Securing the unit
It is only possible to ensure safe operation as a mobile
suction unit by using the special system trolley available
for use with the unit!
The suction unit is placed on the system trolley so that its
feet lock into place in the holes of the unit carrier and it
canbermlyattachedtotheunitcarrierfromunderneath
by means of a knurled screw.
It is imperative that the unit is securely attached to the
system trolley to ensure safe operation and safe travel!
5.0 Options

13
Fig. 15.
Fig. 16.
Fig. 17.
Fig. 18.
5.4 Potential equalisation
(REF 340.0082.0)
A potential equalization connector may be installed on the
system trolley (in the installation groove) as an optional
extra. Installation is performed in accordance with the
attached installation instructions.
5.5 Use of suction unit with disposable
systems
The suction unit may also be used as a tabletop unit with
disposable systems that can be attached to a standard
rail.
5.3 Tray in foot of system trolley
(REF 340.0084.0)
A tray (available as an optional extra) may be inserted in
the foot of the trolley.
5.0 Options
5.6 Battery Backup System (Option)
A Battery Backup (UPS) may be installed on the foot of
the system trolley. Can be usefull to brige over blackout /
brownout. Installation of the board is performed in
accordance with the attached installation instructions.
Make sure to combine the ATMOS C 451 only with Medical
Grade UPS in clinical environment.
Follow the instructions of the user manual of the UPS.
Fig. 19.
Fig. 19.
!
Optionally the suction unit may also be used on the system
trolley with disposable systems that can be attached to a
standard rail.
This requires the standard rail set for installation to the
trolley in accordance with the attached instructions, and
the adapter with standard rail for installation to the securing
device of the suction unit as described in the attached
installation instructions. This seals the upper vacuum con-
nection and provides the opportunity for attaching additional
accessories on the standard rail thus available.
When using the Receptal canisters the following supports
have to be used:
2 x 1,5 l REF 444.0027.0
1 x 2 l REF 444.0030.0
2 x 2 l REF 444.0028.0
1 x 3 l REF 444.0031.0
2 x 3 l REF 444.0029.0

14
6.1 General information on cleaning and disinfection
The measures described herein regarding cleaning
and disinfection do not replace the regulations
valid for operating the device!
The information regarding concentration of
cleaning liquids and disinfectant solutions stated by
the relevant manufacturers must strictly be
observed!
6.0 Cleaning
Medical devices must always offer a maximum in safety and function.
We therefore recommend:
Prior to each application:
if
necessary
Prior to cleaning
6.2 Cleaning and sterilizing the unit
surface
Always disconnect the device from the power line,
before cleaning and disinfecting the surface.
Wipe the surface clean with a cloth soaked in a cleaning
solution or disinfectant. Liquids must not enter the device.
All of the cleaning solutions and disinfectants listed below
can be used.
Should liquids have penetrated into the device, it must
be inspected (danger of an electric shock) and the
pump must be decontaminated resp. exchanged
(infection risk).
6.3 Recommended disinfectants for instruments
Disinfectant Contents (in 100 g) Manufacturer
GIGASEPT FF neu succinic acid dialdehyde 11,0 g Schülke & Mayr, Norderstedt
(Anwendungskonzentrat) dimethoxy tetrahydrofurane 3,0 g
corrosion inhibitors non-ionic tensides
Sekusept aktiv sodiumpercarbonate, phosphonates Ecolab, Düsseldorf
non-ionic tensides
neodisher AN Phosphate > 30 g Dr. Weigert, Hamburg
non-ionic tensides < 5 g
Enzyme
Using the cleaning agent Neodisher AN (manufactured by
Dr. Weigert, Hamburg) cleaning in an automatic cleaner
and disinfecter is also possible.
Thermal disinfection is carried out at 93° C.

15
7.0 Maintenance
6.0 Cleaning
6.4 Recommended disinfectants for surfaces
Disinfectant Contents (in 100 g) Manufacturer
Mikrobac forte benzyl - C12 - C18 - alkyldimeththyl - 19,9 g Bode Chemie, Hamburg
ammoniumchloride
N- (3-Aminopropyl) - N - dodccylpropane- 1,3 - 5,0 g
diamine
Green & Clean SK alkyl-dimethyl-benzyl-ammoniumchloride < 1 g Metasys, Rum (Österreich)
(Anwendungs- dialkyl-dimethyl-ammoniumchloride
konzentrat)
Repairs
The following may require repairs from the manufacturer or an authorized service partner. Prior to sending in the device, please
contact your service partner by phone.
Liquids have penetrated the device
Sudden occurrence of unusual noises
Operational and functional disorders which cannot be resolved by means of the hints describes in the chapter
“Troubleshooting”.
Measures to be taken prior to sending in the device:
If the device has to be sent in for repair after consultation with the manufacturer or an authorized service partner, we ask you to
observe the following:
Please send in the complete device (see scope of delivery).
Please remove all disposable parts and consumables.
Thorough cleaning and disinfection
Airtight packing
Please enclose a detailed error description.
Warranty
ATMOS cannot guarantee an error-free function nor can ATMOS be held liable for damage to people or goods if
non-original ATMOS parts are used,
the information in these operating instructions are disregarded,
assembly,newinstallations,modications,extensionsandrepairsaredonebypeoplewhoarenotauthorisedbyATMOS.
Maintenance
Before putting the device into operation, visually check unit, secretion canister and power cable, accessories, connection cables
and hoses for signs of damage. Damaged cables and hoses must be replaced immediately!
However, every 2 years an inspection and a safety-related check according to EN/IEC 62353 have to be performed.

16
Visually inspect the device, jar and power cord before
each use.
The unit does not require any further maintenance.
8.0 Trouble shooting
The ATMOS C 451 was subjected to a thorough quality control before shipment. If there is, nevertheless, some malfunction,
you possibly might solve this problem yourselves if you observe the following instructions:
Problem Possible cauces Remedy
Unit does not start – Loose power plug – Check connection to supply socket
– no power voltage – Check inbuilding fuse
– Defective fuse – Replace fuse
Insufcientperformance – Leakageswithinthehosesystemorin – Checkcollectionjarlidandhose
or no suction the collection jar lid system, replace sealing ring on
collection jar lid, if necessary
–Filterisclogged – Replacelter,checkllinglevelin
(vacuumgauge indicates a vacuum) collection jar; evacuate jar, if
necessary
– Secretion or blood has been sucked in – Unit has to be returned for repair
and valve plates of the pump are
contaminated
7.1 Change fuse
Remove mains cable.
Press the spring clips of the fuse holder together on
both sides with a small screwdriver and pull out the fuse
holder.
Replace the fuse and push the holder back in until both
spring clips are locked into place.
Then reconnect mains cable.
Fuse REF 008.0749.0
7.0 Maintenance
Fig 21.
Fig 20.
T 1,6 A

17
REF
Accessories for ATMOS C 451
Standard rail set for system trolley
(instead of DDS-docking system for 2 system jars)
340.0081.0
System trolley, basic version for ATMOS C 451 340.0070.0
Potential equalization for system trolley 340.0082.0
Trolley with standard rail 320.0070.1
Trolley with standard rail for
ATMOS C 451 basic device
340.0058.0
DDS standard rail adapter 25 x 10 mm
with vacuum connection for the use of disposable
systems at the unit
340.0059.0
Quick connector for ATMOS C 451 000.0769.0
Optional foot switch for ATMOS C 451
when used as desk-top unit or with trolley
(installation is effected in factory)
340.0060.0
Holder for Battery Backup on request
Battery Backup, Medical Grade on request
9.0 Accessories, consumables and spare parts

18
Description REF
Bellows, silicone rubber................................... 000.0739.0
Fuse 230 V T 0,63 A/H .................................... 008.0634.0
Fuse 115 V T 1,25 A/H..................................... 008.0720.0
Mains cable angle-angle, 5 m.......................... 008.0818.0
Push-in foot for housing................................... 505.0337.0
Fixing screw for
system trolley (star handle) ............................. 000.0726.0
Clampingringforxingscrew ......................... 000.0727.0
Operating instructions...................................... 340.0300.B
9.0 Accessories, consumables and spare parts

19
10.0Technicalspecications
Technical data issued on: 04.07.2011
Airowrateofpump 45 l/min +3/-5 l/min
Max. vacuum -91kPa ( -910 mbar oder 682,5 mmHg)*@ NN
Vacuum readout -1...0 bar ± 16 mbar (Klasse 1,6) ø 63 mm
Additional air regulation mechanical regulating valve
Voltage 230 V~ (+/-10%) 50/60 Hz (REF 340.0300.0)
Current input (max.) max. 0,75 A bei 230 V~
Operating time > 12 h continuous operation without interruption within 24 h
Fuse T 800 mA/H for 230 V~
Heat emission max. 173 J/s
Noise level ≤48dB(A)@1m(ISO7779)beimax.Vakuum
Ambient conditions
Transport/storage
Operation
-22...+122°F
5...90 % humidity,
non-condensing air pressure 700...1060 hPa
+50...+95°F
20...80 % humidity,
non-condensing air pressure 700...1060 hPa
Dimensions HxWxD H 330 x B 240 x T 260 mm
H 1010 x B 360 x T 440 mm (with system trolley)
Weight Ca. 6,7 kg
Regular safety relevant
inspections
Every 2 years
Protection class (EN 60601-1) II
Degree of protection Typ BF
Protection category IPX 1
Classicationacc.toAnnexIXEEC
directions 93/42/EEC
IIa (acc. to EC directive 93/42 EEC)
CE marking CE 0124
Rules applied EN 60601-1: 2007
EN ISO 10079-1: 2000
UMDNS-Code 10 - 217
GMDN-Code 36777
REF 340.0300.0 230 V
* 1 bar = 750,06 mm Hg =1000 hPa / depending on ambient air pressure

20
11.3 Disposal
●The ATMOS C 451 is not comprised of any hazardous materials.
●The materials of the housing can be recycled completely.
●Prior to disposal, device and accessories must be decontaminated.
●The materials are to be separated carefully.
●Payattentiontocountry-specicregulationsfordisposal(e.g.wasteincineration).
11.0 Checking / Reprocessing / Disposal
Disposal within the EC
The suction device described above is a high-quality medical product with a long service life. After its life cycle it must
be disposed of professional. According to the EC directives (WEEE and RoHS) the device may not be disposed of in
domestic waste. Please observe existing national laws and rules for disposal of old devices.
Disposal within the Federal Republic of Germany
In the Federal Republic of Germany the law for electrical devices (ElektroG) rules the disposal of electrical devices.
Since this type of product is mainly used at home for secretion suction in the respiratory tract (after laryngectomy), it
must be assumed that those suction devices could be contaminated. Therefore, this type of device is excluded from
the law for electrical devices. In order to guarantee a proper disposal of your old device, please either pass on your old
device to your specialised dealer or send it directly to ATMOS MedizinTechnik for a professional disposal.
Prior to disposal respectively before transport the device surface must be disinfected.
11.1 Checking ATMOS suction devices
The ATMOS suction devices are maintenance-free in the case they are used according to the operating instructions. Ho-
wever, every 2 years an inspection and a safety-related check according to EN/IEC 62353 have to be performed.
Regular, thoroughly cleaning respectively the operation in line with the operating instructions are assumed.
A regular check of the condensate-controller on the rear side is necessary. Pull out the plastic plug and check the colour at
theendofthehose.Incaseofdiscolouration/depositsamaintenancemeasuremustbeperformedbyacertiedATMOS
service partner!
11.2 Reprocessing
Incasesecretionwassuckedintothedeviceitmaynotbeoperated.ItmustbesenttoanATMOScertiedservicepartner.
Handling of the suction device determines to a large extent its reliability and safety. The hygiene measures described in the
previous chapters are necessary measures for the protection of patients and users, and to maintain functional reliability.
Other manuals for C 451
2
Table of contents
Other Atmos Medical Equipment manuals
Atmos
Atmos C 051 Thorax User manual

Atmos
Atmos LC 27 User manual
Atmos
Atmos Chair M 2 User manual

Atmos
Atmos A 161 User manual
Atmos
Atmos S 61 User manual
Atmos
Atmos S 61 CORIAN integral User manual

Atmos
Atmos Record 55 User manual
Atmos
Atmos E 1 User manual
Atmos
Atmos S 201 Thorax User manual

Atmos
Atmos C 11 Systema User manual
Atmos
Atmos LC 16 User manual

Atmos
Atmos E 341 User manual
Atmos
Atmos S 201 Thorax User manual

Atmos
Atmos Chair 41 Gyne User manual

Atmos
Atmos Chair 21 P User manual

Atmos
Atmos E 2 User manual
Atmos
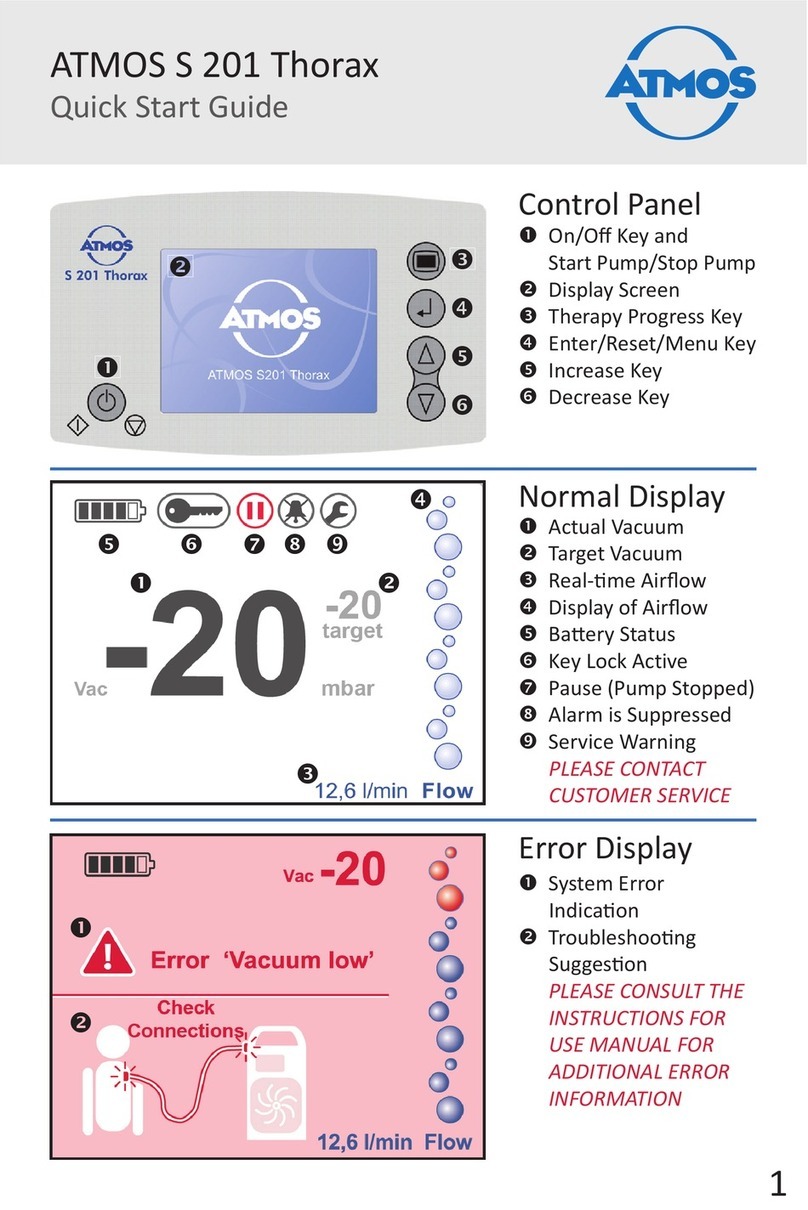
Atmos S 201 Thorax User manual

Atmos
Atmos LC 27 User manual

Atmos
Atmos Strobo 21 LED User manual

Atmos
Atmos C 161 User manual