Bauerfeind uniprox DAHO 2.6 Series User manual
Other Bauerfeind Medical Equipment manuals
Bauerfeind
Bauerfeind GenuTrain S User manual

Bauerfeind
Bauerfeind GenuPoint User manual

Bauerfeind
Bauerfeind GloboPed Mobil User manual

Bauerfeind
Bauerfeind AchilloTrain User manual
Bauerfeind
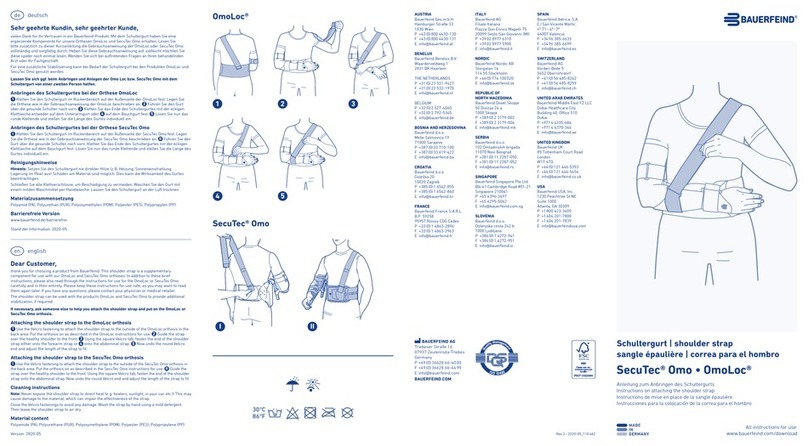
Bauerfeind SecuTec Omo User manual

Bauerfeind
Bauerfeind SecuTec OA User manual

Bauerfeind
Bauerfeind ValguLoc User manual
Bauerfeind
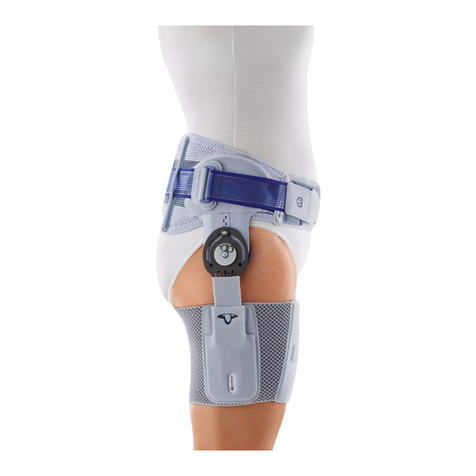
Bauerfeind CoxaTrain User manual

Bauerfeind
Bauerfeind MalleoTrain Plus User manual

Bauerfeind
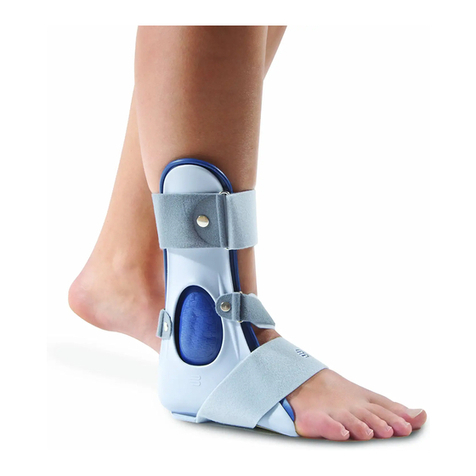
Bauerfeind AchilloTrain Pro User manual
Bauerfeind
Bauerfeind CaligaLoc User manual
Bauerfeind
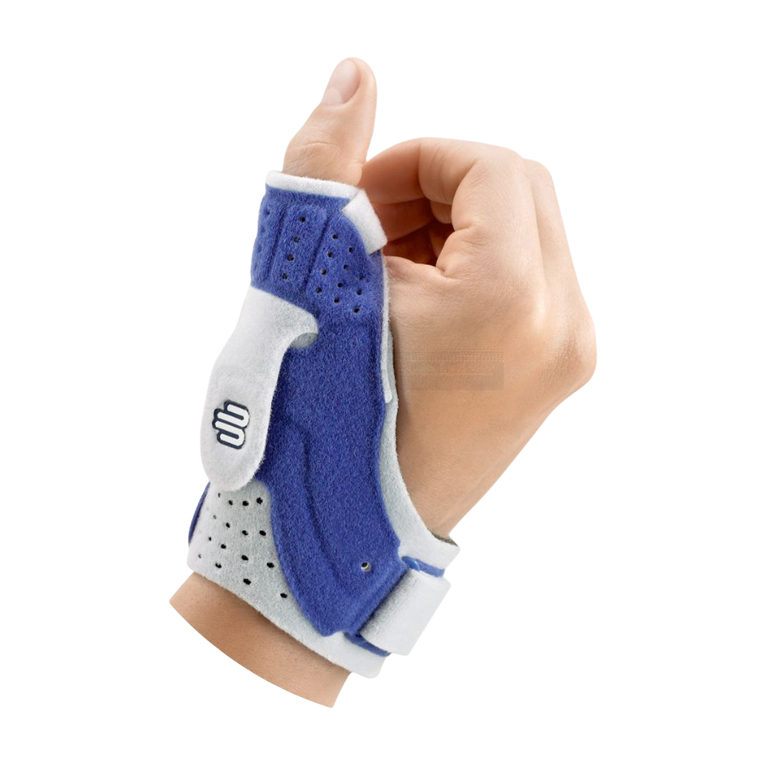
Bauerfeind RhizoLoc User manual

Bauerfeind
Bauerfeind SecuTec OA User manual
Bauerfeind
Bauerfeind MalleoTrain S User manual
Bauerfeind
Bauerfeind SofTec Coxa User manual
Bauerfeind
Bauerfeind CaligaLoc User manual

Bauerfeind
Bauerfeind GloboPed Postop User manual

Bauerfeind
Bauerfeind GloboPed Postop User manual

Bauerfeind
Bauerfeind LumboTrain User manual

Bauerfeind
Bauerfeind ValguLoc II User manual
Popular Medical Equipment manuals by other brands
Nasco
Nasco Life/form LF00995U instruction manual

DENTSPLY
DENTSPLY Triad 2000 Operating and service manual

Rigid Industries
Rigid Industries SeeSnake CS6 Operator's manual

Vitalograph
Vitalograph Compact Instructions for use

DJO Global
DJO Global ARTROMOT-F operating instructions

Sizewise
Sizewise Tom 2 Quick reference guide