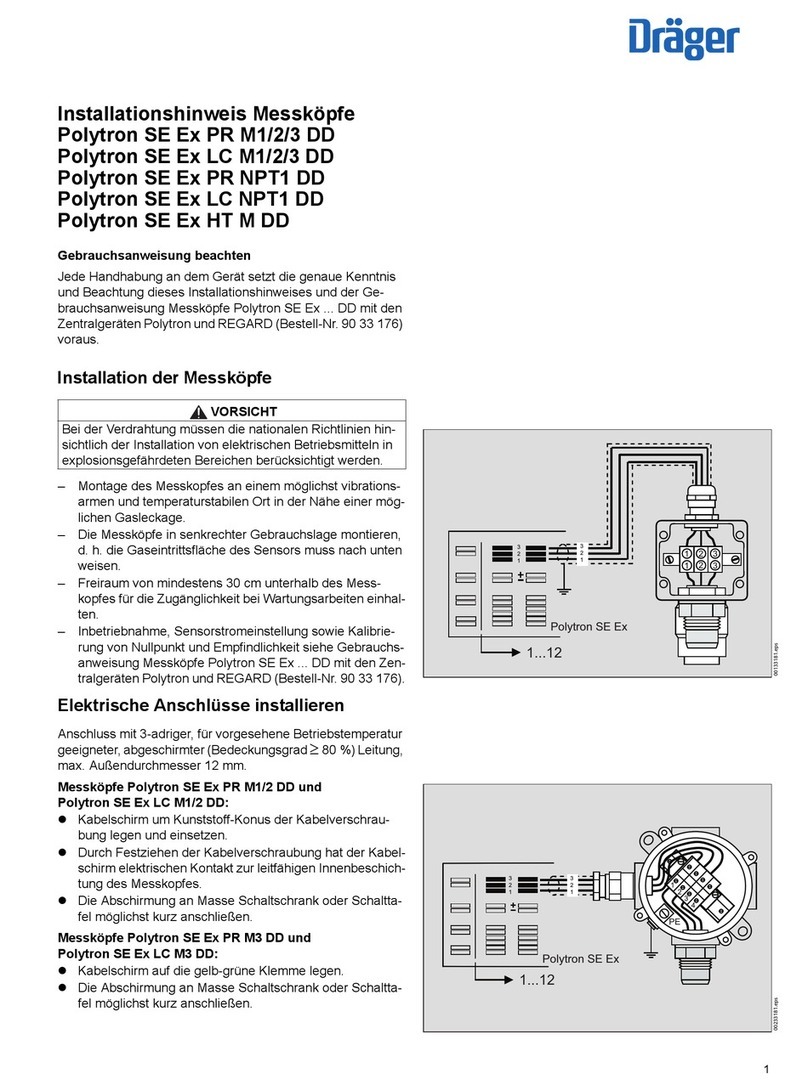
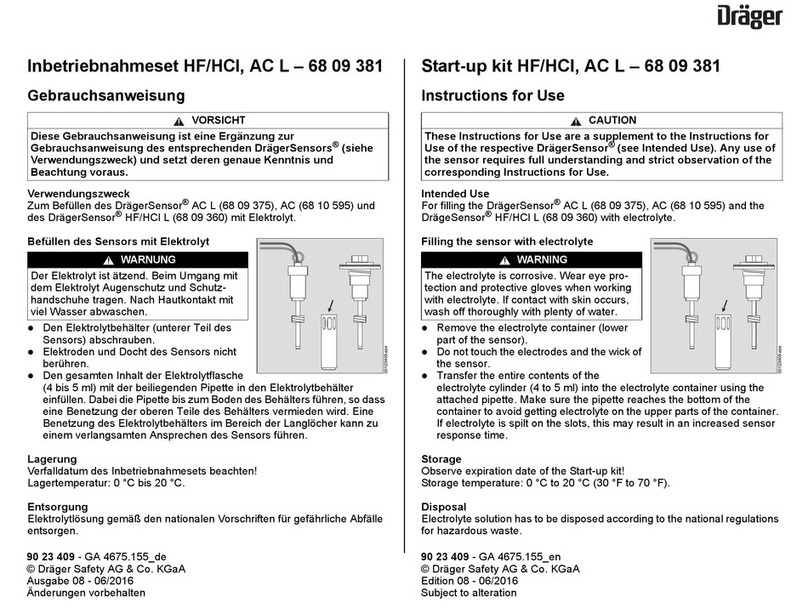
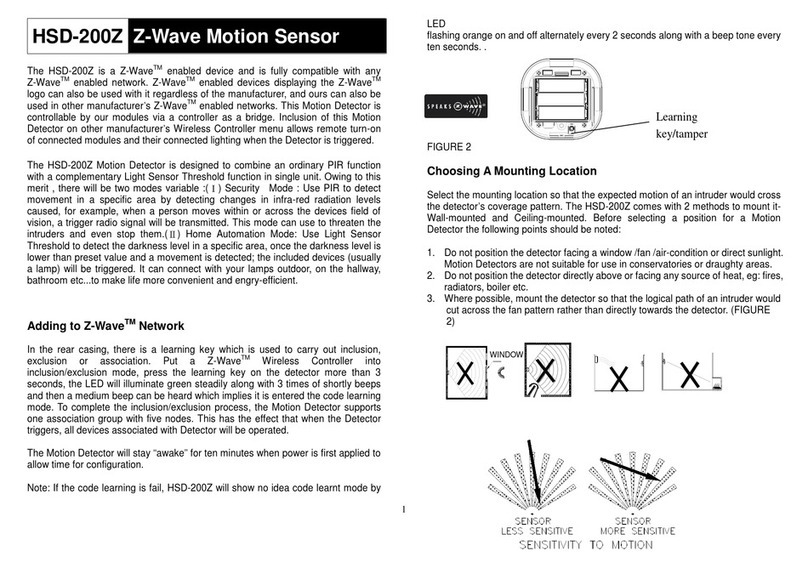
Limitation of Liability
Dräger, Inc.'s liability, whether arising out of or related
to manufacture and sale of the goods, their installation,
demonstration, sales representation, use, performance,
or otherwise, including any liability based upon Dräger,
Inc.'s Product Warranty, is subject to and limited to the
exclusive terms and conditions as set forth, whether
based upon breach of warranty or any other cause of
action whatsoever, regardless of any fault attributable
to Dräger, Inc. and regardless of the form of action
(including, without limitation, breach of warranty,
negligence, strict liability, or otherwise).
THE STATED EXPRESSED WARRANTlES ARE IN
LlEU OF ALL OTHER WARRANTIES, EXPRESSED
OR IMPLIED, INCLUDING, WITHOUT LIMITATION,
WARRANTlES OF MERCHANTABILITY, FITNESS
FOR ANY PARTICULAR PURPOSE, OR
NONINFRINGEMENT.
Dräger, Inc. shall not be liable for, nor shall buyer be
entitled to recover any special incidental, or
consequential damages or for any liability incurred by
buyer to any third party in any way arising out of or
relating to the goods.
Introduction
Operator's Responsibility for Patient Safety
For correct and effective use of the product and in
order to avoid hazards, it is mandatory to carefully
read and to observe all portions of this manual.
The design of the equipment, the accompanying
literature, and the labeling on the equipment take into
consideration that the purchase and use of the
equipment are restricted to trained professionals, and
that certain inherent characteristics of the equipment
are known to the trained operator. Instructions,
warnings, and caution statements are limited, therefore,
largely to the specifics of the Dräger design. This
publication excludes references to various hazards
which are obvious to a medical professional and
operator of this equipment, to the consequences of
product misuse, and to potentially adverse effects in
patients with abnormal conditions. Product modification
or misuse can be dangerous. Dräger, Inc. disclaims all
liability for the consequences of product alterations or
modifications, as well as for the consequences which
might result from the combination of this product with
other products whether supplied by Dräger or by other
manufacturers if such a combination is not endorsed by
Dräger, Inc..
The operators of the incubator system must recognize
their responsibility for choosing appropriate safety
monitoring that supplies adequate information on equip-
ment performance and patient condition. Patient safety
may be achieved through a wide variety of different
means ranging from electronic surveillance of
equipment performance and patient condition to simple,
direct observation of clinical signs. The responsibility
for the selection of the best level of patient monitoring
lies solely with the equipment operator.
Introduction Operator's Responsibility for Patient Safety
Limitation of Liability
4
Operating Instructions Incubator 8000 IC