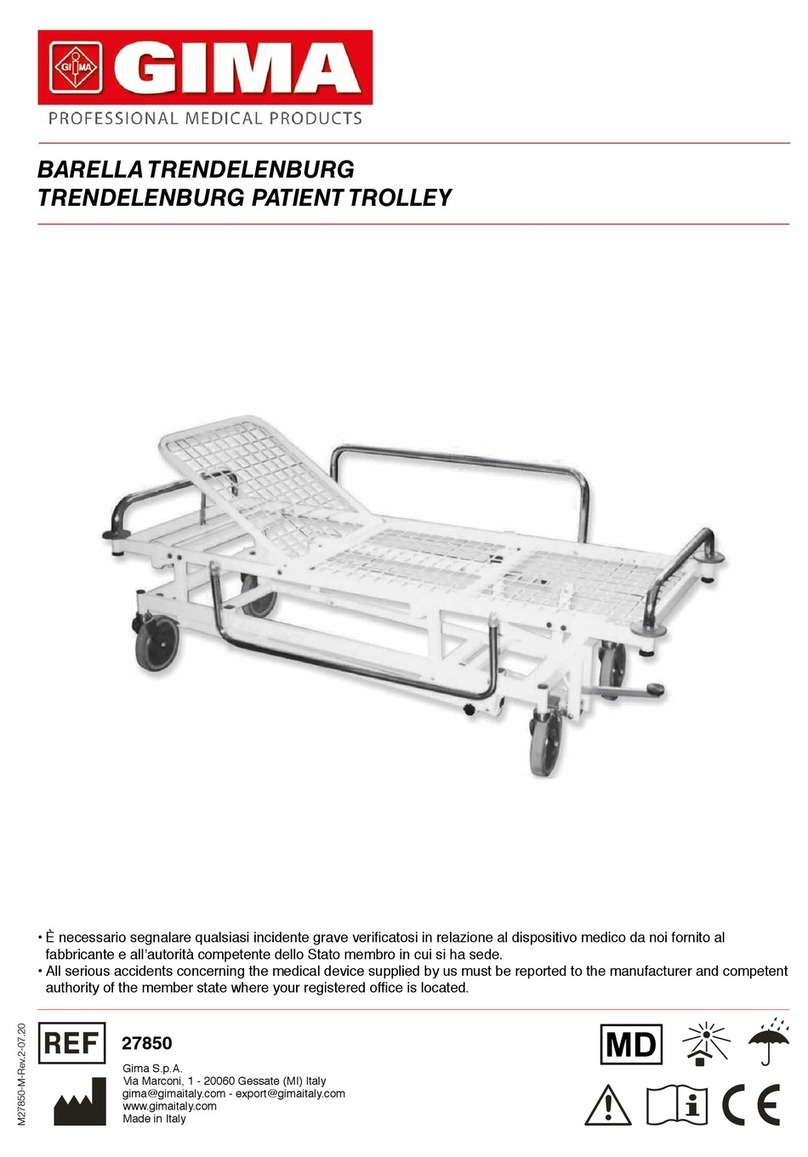
15 ENGLISH
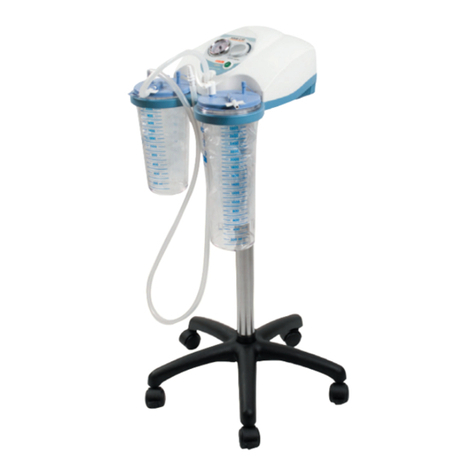
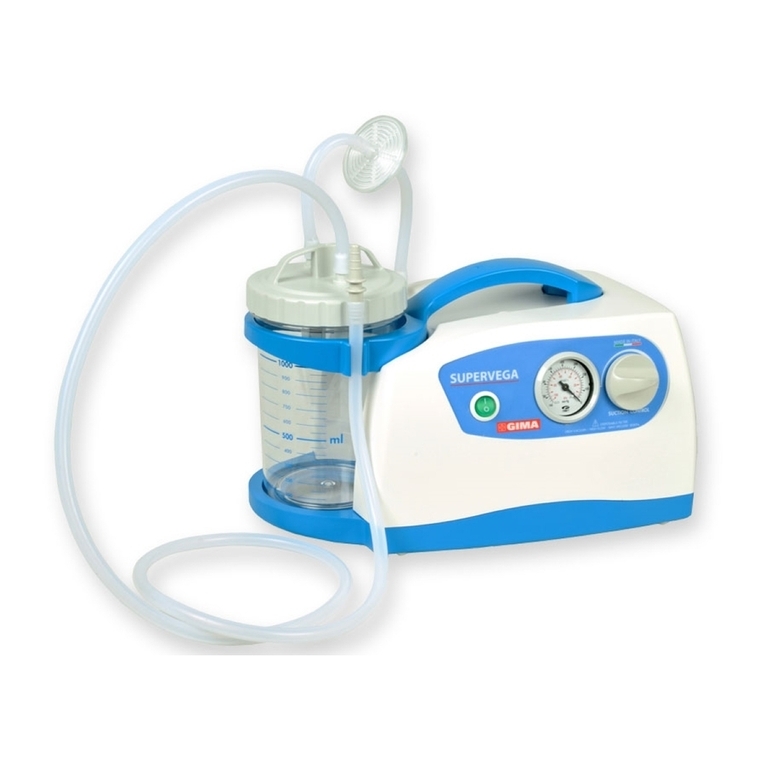
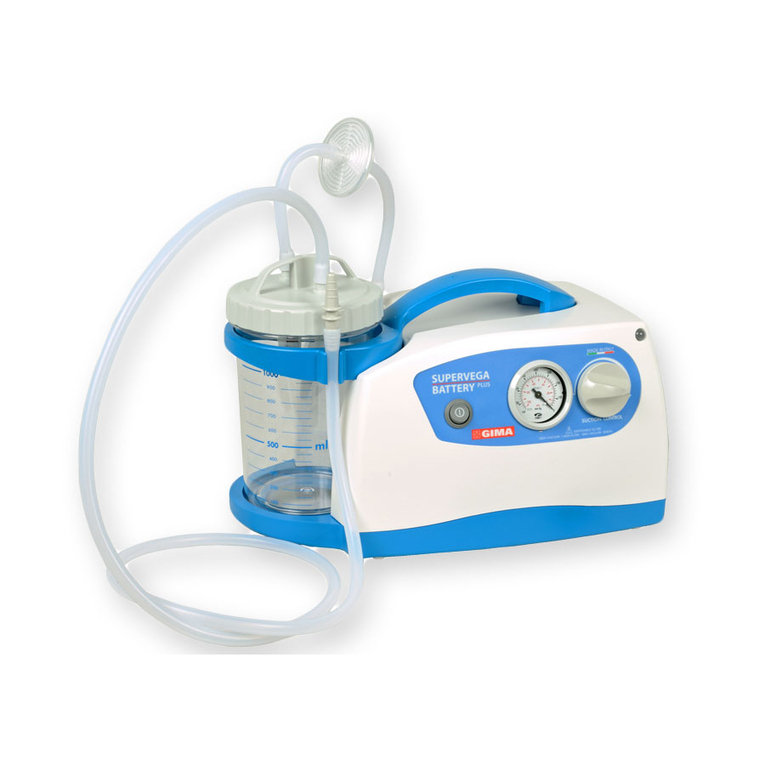
SUPER VEGA 36 SURGICAL SUCTION PUMP is a suction pump particularly suited for use in hospital
wards, in patients with tracheotomy, in small surgical applications and post-operative treatments at home. A
device that can be used for nasal, oral and tracheal aspirations in adults or for body liquids in children (for
example mucus, phlegm and blood). A device designed to offer ease-of-transport and almost continuous use
thanks to the adoption of an electronic system to manage the power supply. Supplied with an audible alarm
and visual tell-tale (luminous LED) to indicate the battery status. Featuring a body in plastic with high thermal
and electrical insulation in compliance with recently introduced European safety regulations. Supplied com-
plete with sterilizable polycarbonate jug with overow valve. Features a suction regulator and vacuum gauge
located on the front panel.
GENERAL WARNING
Read instruction manual carefully before use. The device is for use by qualied personnel (surgeon / profes-
sional nurse / assistant) the use of the device at home is restricted to an adult in full possession of mental
faculties and / or home carers the instrument must not be disassembled. For technical service always contact
Gima S.p.A.
IMPORTANT SAFETY RULES
1. Check the condition of the unit before each use. The surface of the unit should carefully be inspected for
visual damage. Check the mains cable and do not connect to power if damage is apparent;
2. Before connecting the appliance always check that the electric data indicated on the data label and the type
of plug used, correspond to those of the mains electricity to which it’s to be connected;
3. Respect the safety regulations indicated for electrical appliances and particularly:
- Use original components and accessories provided by the manufacturer to guarantee the highest ef-
ciency and safety of the device;
- The device can be used only with the bacteriological lter;
- Never immerge the appliance into water;
- Do not place or store the aspirator in places where it may fall or be pulled into the bathtub or washbasin.
In the event it is accidentally dropped, do not attempt to remove the device from the water whilst the plug
is still connected: disconnect the mains switch, remove the plug from the power supply and contact the
GIMA technical service department. Do not attempt to make the device work before it has been thorough-
ly checked by qualied personnel and/or the GIMA technical service department.
- Position the device on stable and at surfaces in a way that the air inlets on the back aren’t obstructed;
- To avoid incidents, do not place the aspirator on unstable surfaces, which may cause it to accidentally fall
and lead to a malfunction and/or breakage. Should there be signs of damage to the plastic parts, which
may expose inner parts of the energised device, do not connect the plug to the electrical socket. Do not
attempt to make the device work before it has been thoroughly checked by qualied personnel and/or the
GIMA technical service department.
- Don’t use in the presence of inammable substances such as anaesthetic, oxygen or nitrous oxide;
- Don’t touch the device with wet hands and always prevent the appliance coming into contact with liquids;
- Don’t leave the appliance connected to the power supply socket when not in use;
- Don’t pull the power supply cable to disconnect the plug remove the plug from the mains socket correctly;
- Store and use the device in places protected against the weather and far from any sources of heat. After
each use, it is recommended to store the device in its own box away from dust and sunlight.
- In general, it is inadvisable to use single or multiple adapters and/or extensions. Should their use be
necessary, you must use ones that are in compliance with safety regulations, however, taking care not to
exceed the maximum power supply tolerated, which is indicated on the adapters and extensions.
- Prevent children from using the device without proper supervision;
- Never leave the appliance near water, do not immerse it in any liquid. If the device has fallen into water,
unplug it before you hold it. Do not use the appliance if the plug or AC / DC power supply is damaged or
wet (send it immediately to an authorized service center or technical service).
4. For repairs, exclusively contact technical service and request the use of original spare parts. Failure to
comply with the above can jeopardise the safety of the device;
5. Use only for the purpose intended. Don’t use for anything other than the use dened by the manufacturer.
The manufacturer will not be responsible for damage due to improper use or connection to an electrical
system not complying with current regulation.