3
www.icaretonometer.com
the levels permitted by the relevant standards, they may cause interference
in other, nearby devices, for example sensitive sensors.
• If you do not use the tonometer for a long time, remove the batteries, as
they may leak.
• Be sure to dispose of the single-use probes properly (for example, in a mixed
waste bin).
• Batteries, packaging materials and probe bases must be disposed of
according to local regulations.
• Make sure you use batteries with built-in PTC protection, for example
Energizer Lithium Photo 123 3V CR123A.
• Do not cover the eye recognition transmitters or sensor during the
measurement, for example with your fingers. Keep your hand, hair etc.
and objects such as pillows away from the temple side of your eye, as they
produce an infrared reflection that causes an error.
• The tonometer turns off automatically after 3 minutes if you do not use it.
• Update the tonometer's time to your local time. It is done automatically
byperforming the steps 1 and 2 under section 12. Reading the
measurement data.
• Check that the silicone lid covers the USB port during the measurement.
• The measurement method of the Icare HOME tonometer is based on
magnetic induction and therefore an external magnetic field in line with
the probe may prevent the measurement. In such case the tonometer
will continuously ask to repeat the measurement. Situation can be solved
either by removing the source of interference from the vicinity of the
deviceor by performing the measurement in different location with no
suchinterference.
2. GLOSSARY
Tonometer= a device for measuring your IOP
mmHg= units of measurement for the pressure in your eye
Position tag= support positions for your eyes are written on it
Probe= tonometer's single-use item that lightly touches your eye
Probe base light= light ring that assists you to position the probe
Shelf life= the time the probe remains sterile in its intact packing
Cornea= the eye's outermost dome shaped clear layer
Forehead/cheek supports= tonometer's adjustable supports
Expected service life= expected life time before replacement
3. INDICATIONS FOR USE
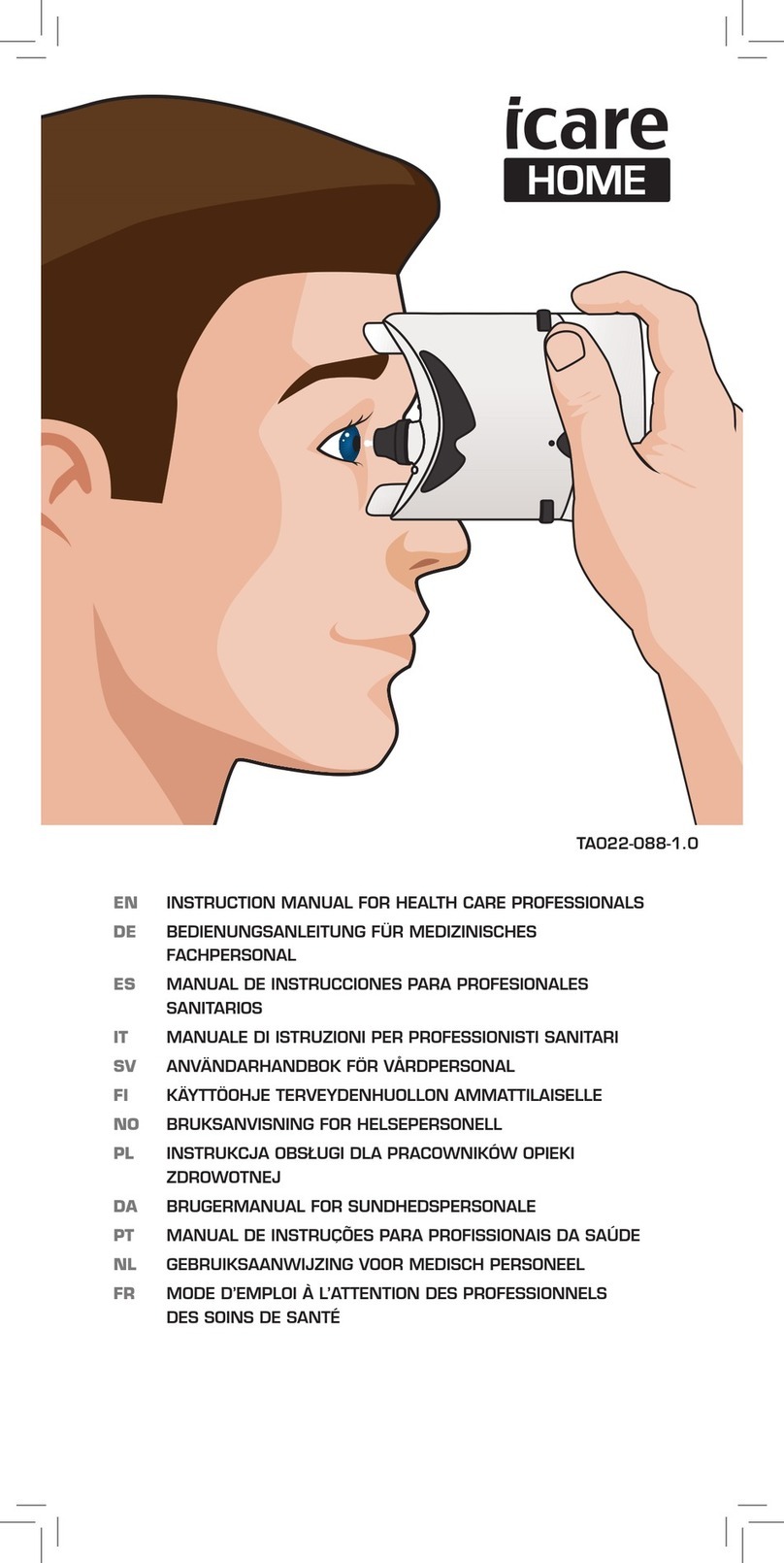
The Icare HOME tonometer is intended for monitoring of intraocular pressure
(IOP) of the human eye. It is indicated for use by patients or their caregivers
under supervision of an eye care professional.
4. HOW DOES THE TONOMETER WORK
The tonometer measures your eye pressure using a disposable probe that
gently contacts your eye during the measurement. You may need a mirror to
help you determine the correct measurement position. The recommended
frequency of measurements is according to the healthcare professional's
instruction or 3-6 times per day.
A complete measurement is a series of six very rapid measurements. It may
take from several seconds to a minute to take the measurement. The probe
moves to the cornea and back during each of the six rapid measurement.
After the series of six measurements, the tonometer calculates your final
eyepressure and stores it in the tonometer's memory.
The probe is disposable. You can use the same probe for both eyes if the
health care professional instructed you to measure both eyes. After you have
taken the measurements for both eyes place the probe back in its container
and dispose of it in a mixed waste bin.
The tonometer includes infra-red eye recognition sensors to identify which eye,
right or left, you are measuring. It is important not to cover these sensors with
your fingers, hand, hair etc., because covering the sensors causes an error.
Itisalso important to keep objects such as pillows away from the temple side
of your eye, as they produce an infrared reflection that causes anerror.
Do not take the device into use before you have been trained in its use.
Donot modify or discontinue your treatment plan without receiving guidance
from a health care professional.