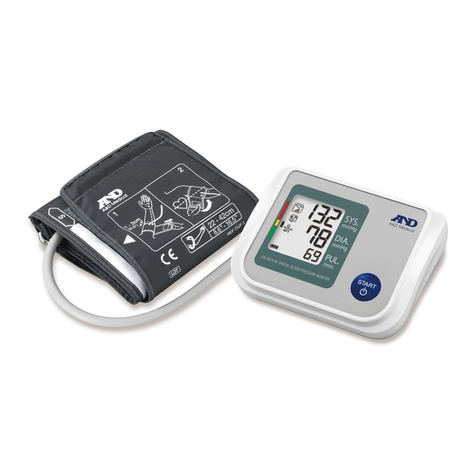
Nissei DSK-1031 User manual

DSK-1031 INSTRUCTOIN MANUAL
DIGITAL BLOOD PRESSURE MONITOR DSK-1031
INSTRUKCJA OBSŁUGI
CIŚNIENIOMIERZA CYFROWEGO DSK-1031
РЪКОВОДСТВО ЗА ЕКСПЛОАТАЦИЯ
НА ЦИФРОВ ТОНОМЕТЪР DSK-1031
UŽIVATELSKÝ NÁVOD
K DIGITÁLNÍMU TONOMETRU DSK-1031
A DSK-1031 TÍPUSÚ DIGITÁLIS TONOMÉTER
HASZNÁLATI UTASÍTÁSA
MANUAL DE UTILIZARE
A TENSIOMETRULUI DIGITAL DSK-1031
ROU HUN CZE BGR POL ENG
2
ENG
GENERAL INFORMATION
INDICATIONS FOR USE
This Instruction Manual is designed to assist the user with safe and effective operation of
the digital blood pressure monitor DSK-1031 (hereinafter – the “Device”). The device is de-
signed to measure systolic and diastolic blood pressure and heart rate reading in patients
aged 13 years and older. This device should not be used for neonate or infant. Consult your
doctor for blood pressure measurement in children or person in pregnancy or under pre-
eclamptic condition. The device is recommended for use in patients with unstable (non-
permanent) blood pressure or hypertension at home as a supplement to medical surveil-
lance. The cuff is suitable for a upper arm with a circumference of about 22 - 42 cm.
Blood
pressure is measured in the range from 50 to 250 mmHg for systolic and 40 to 180
mmHg for diastolic, in the range from 40 to 160 heartbeats per minute.
OPERATION PRINCIPLES
The device uses the oscillometric method of measurement. The cuff connected to the
electronic module is wrapped around the upper arm.
When you press the START/STOP button, the device starts the automatic inflation,
which is followed by the blood pressure measurement. The sensor element of the de-
vice detects slight pressure oscillations in the cuff produced by the expansion and
contraction of the brachial artery in response to each heartbeat. Inflation is stopped
when the cuff is pumped enough to determine diastolic and systolic pressure, where-
upon the air is discharged from the cuff. Rhythm and amplitude of the pressure waves
are measured and displayed on the LC display as a numerical value in millimeters of
mercury. The device has an arrhythmia indicator, as well as two memories with 60
cells in each calculating the average value.
NISSEI New Technologies
Fuzzy Inflation Algorithm – is an algorithm for automatic selection of the
cuff inflation pressure. Using this algorithm, the device by itself determines
the pressure level to which it is necessary to inflate the cuff based on the
patient’s systolic pressure. Owing to the Fuzzy Inflation algorithm the device
becomes easier to use, while the measurement gets more comfortable and
more accurate.
Arrhythmia indicator is a special icon on the display that informs on the
irregular heartbeat, while the measurement result is correct.
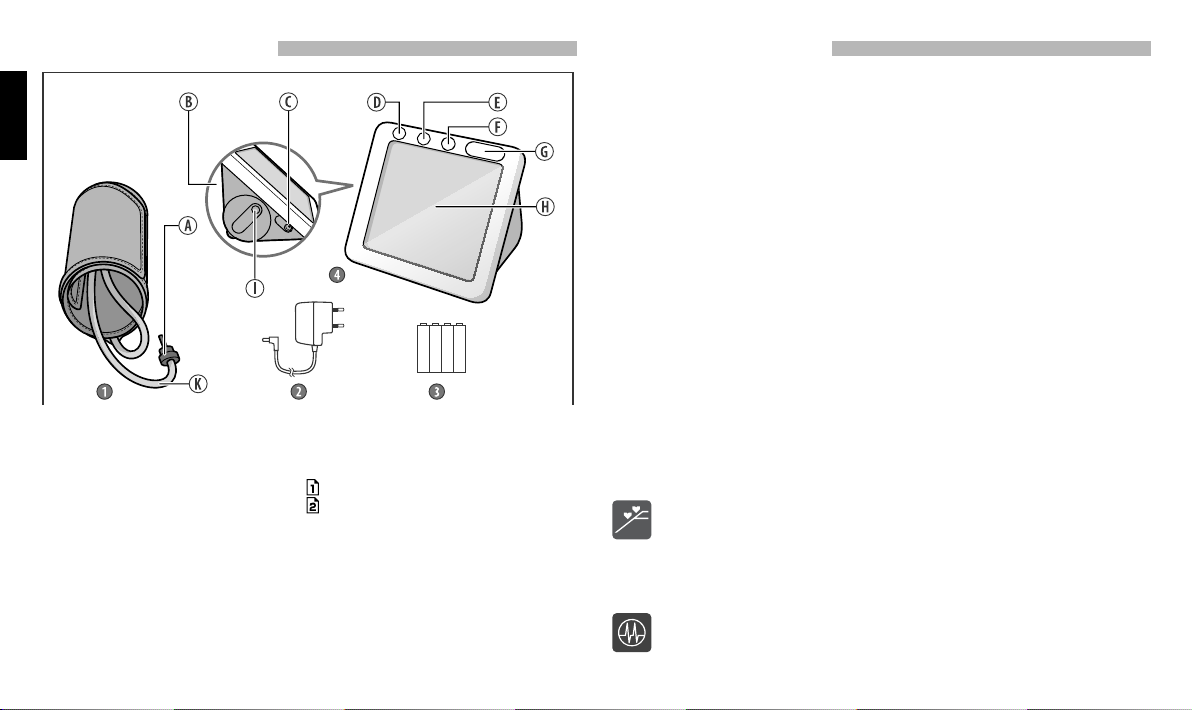
PARTS AND COMPONENTS
1. CUFF
2. AC ADAPTOR
3. BATTERIES
4. ELECTRONIC UNIT
A. AIR PLUG
B. BATTERY COMPARTMENT
C. AC ADAPTOR JACK
D.
SET BUTTON (SETTINGS)
E. BUTTON (MEMORY 1)
F. BUTTON (MEMORY 2)
G. START/STOP BUTTON (START/STOP )
H. LCD DISPLAY
I. AIR CONNECTOR
K. AIR TUBE

3
ENG
crease in pressure values. Therefore, blood pressure measured at home is often differ-
ent from that measured in the clinic. Since blood pressure increases at low tempera-
tures, measurements should be made at room temperature (about 20°C). In case the
product is stored in the environment with ambient temperature above 40˚C or below
10˚C, please leave it for at least 2 hours before taking a measurement. During the day,
the difference in the readings in healthy people may attain 30-50 mm Hg for systolic
(upper) pressure and up to 10 mm Hg for diastolic (lower) pressure. Dependence of
blood pressure on various factors is individual for each person. Therefore it is recom-
mended to keep a special recording of blood pressure readings. ONLY A DOCTOR MAY
ANALYZE TRENDS IN CHANGING YOUR BLOOD PRESSURE BASED ON CORRESPONDING
RECORDINGS.
4. In case of cardiovascular diseases and a number of other diseases that require
the blood pressure monitoring, measurements should be carried out in the hours
specified by a doctor. REMEMBER THAT THE DIAGNOSTICS AND ANY TREATMENT OF
ARTERIAL HYPERTENSION SHOULD BE CARRIED OUT ONLY BY A DOCTOR BASED ON
BLOOD PRESSURE READINGS OBTAINED BY A DOCTOR. MEDICAL DRUG ADMINISTRA-
TION OR CHANGE OF DOSAGES SHOULD BE MADE ONLY BY PRESCRIPTION OF AN AT-
TENDING DOCTOR.
Blood pressure variations during a day
Systolic
Diastolic
Arterial pressure (mmHg)
Time of day
Fig.1
5. In case of disorders such as deep vascular sclerosis, weak pulse wave and break in
rhythm of heart contractions, the correct blood pressure measurement can be com-
plicated. IN THIS CASE, A DOCTOR SHALL PROVIDE RECOMMENDATIONS IN RELATION
TO USE OF THIS DEVICE.
6. KEEP QUIET DURING THE MEASUREMENT TO OBTAIN THE CORRECT BLOOD PRES-
SURE READING WHEN USING THE ELECTRONIC DEVICE. The blood pressure measure-
Touch control – is control of the device by slight touching it.
Detection of interference noises – indicator informs on the occurrence of
noises that can affect the measurement result.
Control of correct fixing the cuff – shows if the cuff is fastened too tight
or too loose.
Indication of reliability – this symbol is displayed when all stages of
proper measurement procedure are observed.
Pulse pressure – along with the measurement result the device displays
the pulse pressure value. Pulse pressure is a difference between systolic
and diastolic pressure.
WARNING! Using a cuff that is different from that supplied with this device is not allowed.
COMPLETE SET
The complete set includes
- Electronic unit - 1 pc.
- Cuff (including air hose and air hose plug) - 1 pc.
- Batteries - 4 pcs.
- Power supply source - 1 pc.
- Bag - 1 pc.
- Instruction Manual - 1 pc.
- Warranty - 1 pc.
- Packaging - 1 pc.
RECOMMENDATIONS ON CORRECT MEASUREMENTS
1. If treated with hemodialysis or anticoagulants, antiplatelets or steroids, refer to
your doctor about the blood pressure measurement.
2. Malfunctions are possible when the device is used near working mobile phones,
microwave ovens and other equipment generating electromagnetic radiation.
3. For correct measurement it is necessary to know that the BLOOD PRESSURE IS SUB-
JECT TO SHARP FLUCTUATIONS EVEN IN SHORT TIME INTERVALS. The blood pressure
level depends on many factors. It is commonly lower in summer and higher in winter.
Blood pressure varies along with atmospheric pressure and depends on the physical
exertion, emotional excitability, stress and diet. Medical drugs, alcohol and smoking
exert great influence as well. Occasionally, measurements in the clinic cause an in-
4
ENG
ment should be carried out in a quiet comfortable atmosphere at room temperature.
Exclude meal an hour before the measurement, and exclude smoking, soft drinks, and
alcohol 1.5-2 hours before the measurement.
7. Accuracy of the blood pressure measurement depends on matching the device
cuff and size of your arm. THE CUFF SHOULD NOT BE TOO SMALL OR TOO BIG.
8. Repeated measurements are carried out at 5-minute intervals to recover the blood
circulation. However, persons suffering from severe atherosclerosis, due to a significant
loss of elasticity of blood vessels, need longer intervals between measurements (10-15
minutes). This also concerns patients suffering from long-term diabetes. For more ac-
curate determination of blood pressure it is recommended to carry out a series of three
consecutive measurements and to calculate the average value of measurement results.
9. Do not use this device in an explosive environment such as near flammable anes-
thetics or inside oxygen chamber.
10. The system may fail to yield specified measurement accuracy if operated or stored
in temperature or humidity conditions outside the limits stated in the specifications
section of this manual.
11. Do not use cuffs or accessories other than those specified by the manufacturer.
Otherwise, correct measurement readings cannot be obtained.
12. Do not apply the cuff over wounded arm, arm under an intravascular access or
therapy or an arterio-venous shunt, or arm on the side of a mastectomy or lymph
node clearance. Otherwise injury may be resulted.
13. Make sure that inflation of the cuff is not causing prolonged impairment of blood
circulation. Also, be cautious about temporary loss of the functions of any other medi-
cal equipment if any monitoring equipment is used on the same limb with the blood
pressure measuring cuff.
14. To avoid harmful injury due to interfered blood flow from cuff inflation, make sure
that AIR HOSE is not kinking before measurement. Otherwise, cuff inflation may not
be conducted properly and prolonged.
15. Do not take out batteries or unplug the AC adaptor when the device is turned on.
Make sure to switch off the device before removing batteries or AC adaptor.
16. Do not touch the output plug of AC adaptor during measurement.
17. Do not inflate the cuff when it is not wrapped around your arm.
18. Do not apply the cuff on the limb which the intravenous drip infusion is imple-
mented.
POWER SUPPLY OF THE DEVICE
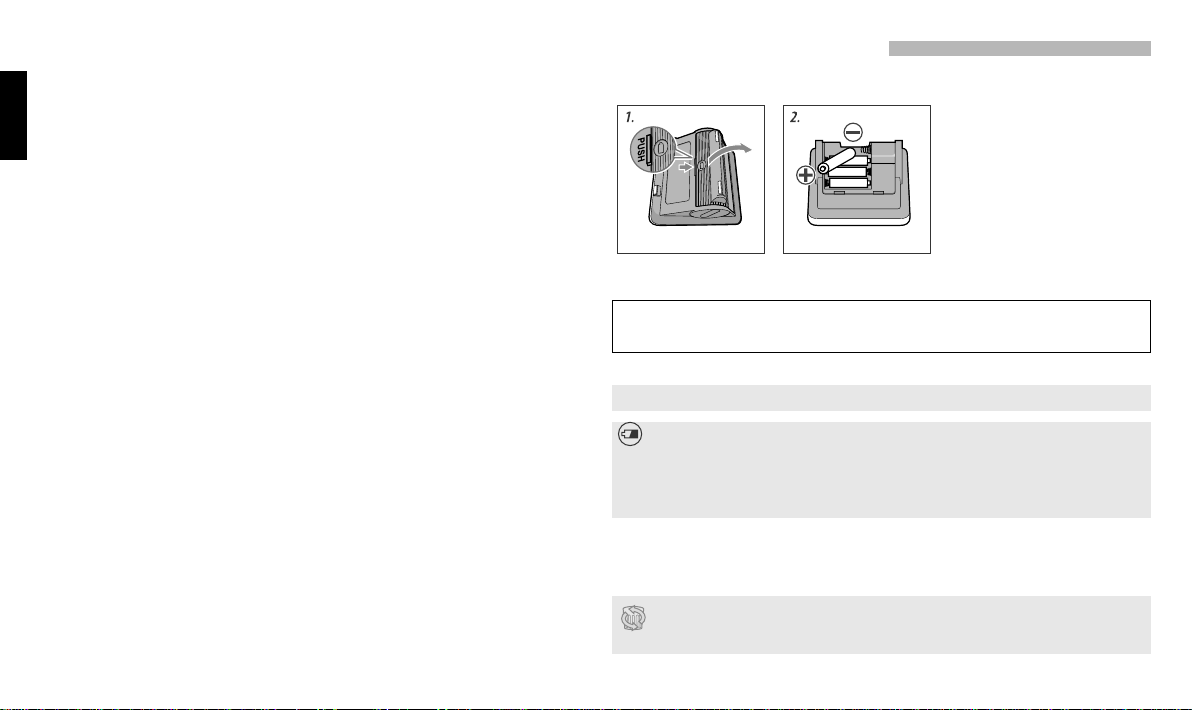
INSTALLATION OF BATTERIES
Fig.2 Fig.3
1.
Open the battery compart-
ment (Fig. 2).
2. Install four “AA” batteries in
the compartment.
Make sure that polarity corre-
sponds to signs (+) and (-) shown
inside the compartment (Fig. 3).
Batteries are readily installed
by pressing the end “-“ on the
spring.
Allowed to rechargeable batteries. To charge the batteries, use the special charger
(not included).
3. Close the battery compartment. Do not use excessive force when removing the cover.
Do not use excessive force when removing the cover.
Battery Replacement Indicator
Replace all the batteries when the battery replacement indicator is flashing on
the display during the measurement. If upon the device turning on the indicator
is steadily flashing, the measurement will not be possible until all the batteries are
replaced. The battery replacement indicator does not show a discharge degree.
Use alkaline batteries to increase the device operation duration. Ordinary zinc-carbon
batteries require more frequent replacement. The enclosed batteries are meant for
testing the sold device, and their operation period can be less than that of batteries
acquired in the trade network.
Since neither the device nor the batteries are the waste that can be utilized at home,
follow your national/local regulations for waste recycling and take them to corre-
sponding collection facilities.
5
ENG
USE OF THE DEVICE WITH THE POWER SOURCE
Socket for the power source is arranged on the left side of the device. To use the device
with the power source, connect the power source connector to the device, install the
power source plug into the socket outlet, and press the «START/STOP» button.
When finished, turn off the device by pressing the «START/STOP» button, unplug the
power source from the socket outlet and disconnect the power source connector
from the device.
NOTE! If there is no battery in the device, turning off the power source will result in
zeroing of measurement results stored in the device memory and set date and time. If
you do not want to make the data erased, do not remove the batteries from the device
when using the power source.
SETTING DATE AND TIME
Date and time can be set after installing batteries. Setting the date and time guarantees
the preservation of measurement results with indicated correct date and time. The de-
vice can be used without setting the date and time.
Press and hold the SET button until the display flashes the value of the year.
Date and time are set in the following order: year, month, day, hour and minute.
1. Setting the Year
Use the button to increase and button to decrease the year value. Press the SET
button to confirm and to pass to the next step.
2. Setting the Month
Use the button to increase and button to decrease the month value. Press the
SET button to confirm and to pass to the next step.
3. Setting the Day
Use the button to increase and button to decrease the day value. Press the SET
button to confirm and to pass to the next step.
4. Setting the Time
Watch uses a 12-hour time format of day. Use the button to increase and button
to decrease the hour or minute value. Press the SET button to confirm the settings. To
stop the setting, press the «START/STOP» button.
IMPORTANT! If the date and time are set, then when turned off the device display will
show the current time.
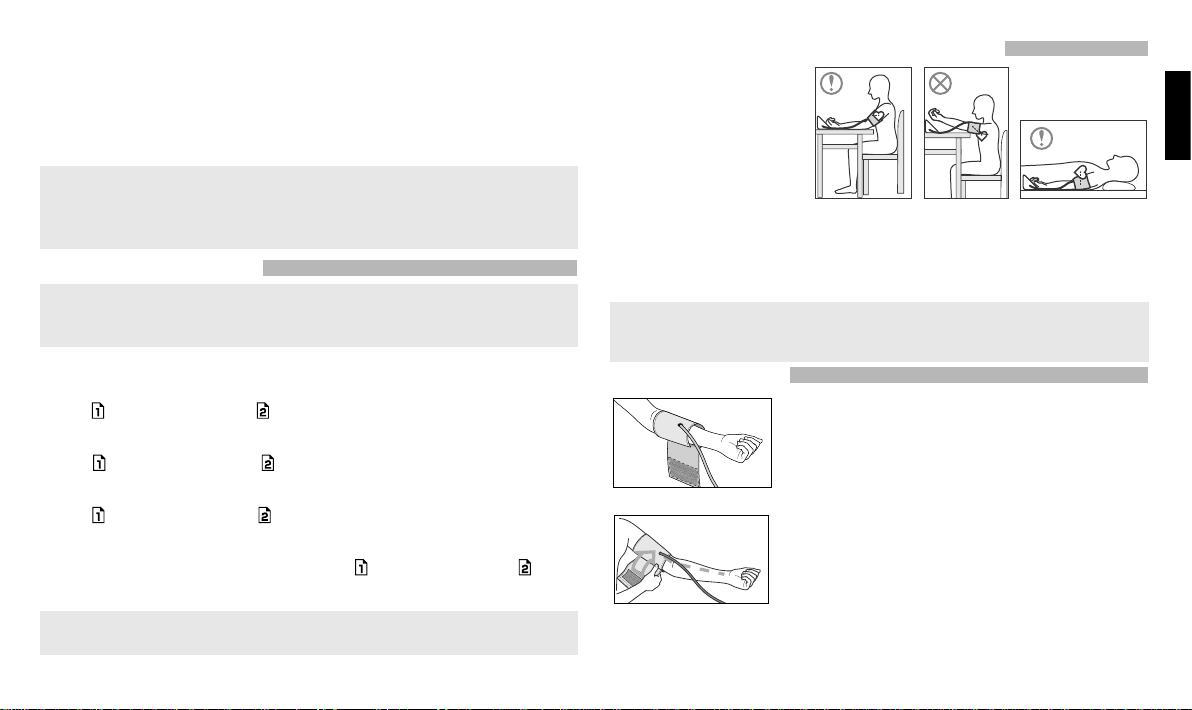
CORRECT POSITION DURING MEASUREMENT
Fig.4 Fig.5 Fig.6
Sit at the table, let the chair and
table support your back and
arm, and keep your feet flat on
the floor as you take the blood
pressure measurement (Fig. 4).
Make sure that the measure-
ment location on the upper
arm is at approximately the
same height as the heart, and
that the forearm is extended naturally on the table and does not move.
You may lie on your back and take the measurement (Fig.6). Look at the ceiling, stay calm,
and do not move your neck or body during the measurement. Again, make sure that the
measurement location on the arm is at approximately the same height as the heart.
Measured values may vary slightly, depending on the position during the
measurement. If the cuff is above/below the level of the heart, resulting reading
may be incorrect (lower/higher)
CUFF PREPARATION
Fig.7
1. Apply the cuff to your left upper arm so that the air
tube is directed to your palm
(Fig. 7)
. If the measurement
on your left arm is difficult, you may use your right arm. In
this case remember that the readings may differ by 5-10
mmHg and even more.
Fig.8
2.
Wrap the cuff around your upper arm so that the bottom
of the cuff is approximately 2-3 cm above your elbow. Air
tube should be directed towards the palm (Fig. 8).
Other manuals for DSK-1031
3
Table of contents
Other Nissei Blood Pressure Monitor manuals
Nissei
Nissei DS-10 User manual

Nissei
Nissei DS-137 User manual

Nissei
Nissei DS-400 User manual

Nissei
Nissei WS-1011 User manual

Nissei
Nissei WSK-1011 User manual
Nissei
Nissei ds-1902 User manual

Nissei
Nissei WS-1300 User manual

Nissei
Nissei WSK-1011 User manual

Nissei
Nissei DSK-1031 User manual

Nissei
Nissei DSK-1031 User manual

Nissei
Nissei WS-820 User manual

Nissei
Nissei DSK-1031 User manual

Nissei
Nissei DS-S10 User manual

Nissei
Nissei DS-B10 User manual

Nissei
Nissei DS-10a User manual

Nissei
Nissei DS-1011 User manual

Nissei
Nissei DSK-1011 User manual

Nissei
Nissei DSK-1011 User manual

Nissei
Nissei DS-10 User manual

Nissei
Nissei DS-500 User manual