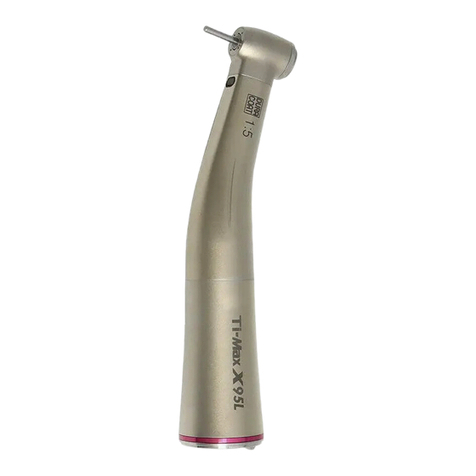
7
ENGLISH GERMAN FRENCH SPANISH ITALIAN
CAUTION
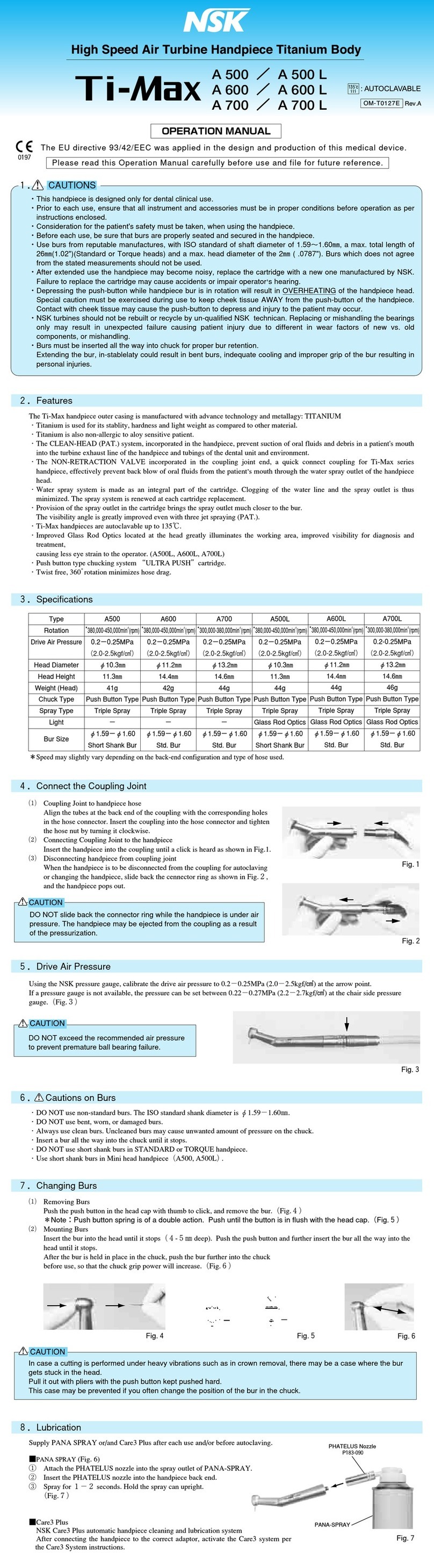
• Make sure to align the " " mark with " " mark. If those marks are not completely aligned, it may cause handpiece overheating or patient
burn injuries.
• Always insert the bur fully into the chuck. Insufficient insertion of the bur could cause premature failure of the bearing.
• Remove the bur only after the handpiece has completely stopped rotating.
• Do not rotate the handpiece when the Bur Lock Ring is turned to " " (OPEN) position or a bur is not inserted.
• Always keep the bur shank clean. Entry of debris into the chuck, via the bur shank, could cause bur rotation slip and also prevent the bur from
being securely located in the chuck.
• Do not exceed the bur speed recommended by the bur manufacturer.
• Do not exceed maximum bur length recommended by the handpiece manufacturer.
• Do not apply excess pressure to the bur as it may break or bend or become difficult to remove.
• DO NOT use burs with problems listed below as the bur may break or disengage from the chuck.
- Bent, deformed, anisomerous (worn), rusted, broken, deficient bur.
- Bur which is cracked on the edge or axis.
- Non-ISO standard, or tampered bur