CE labelling
This product satisfies the requirements of the applicable European and national directives
3. Intended use
Intended use
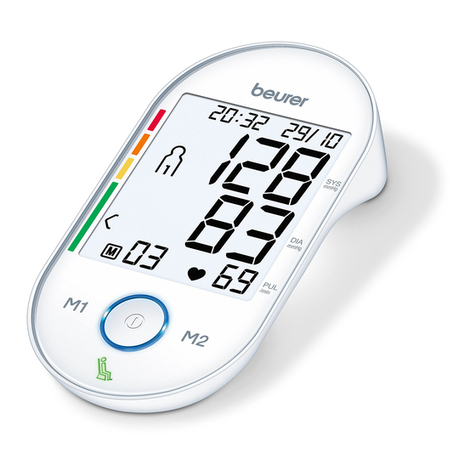
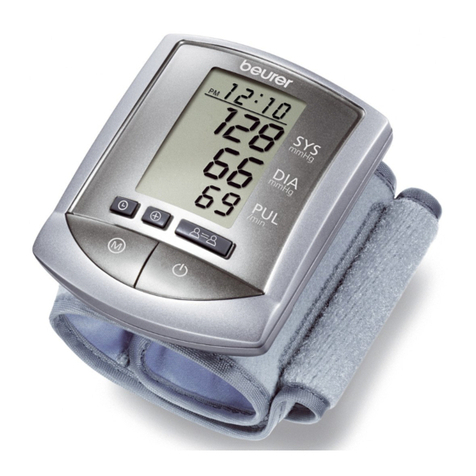
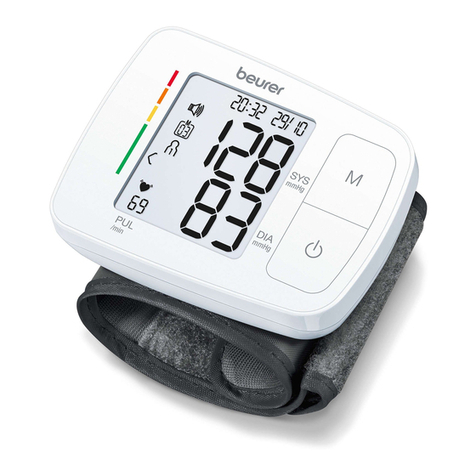
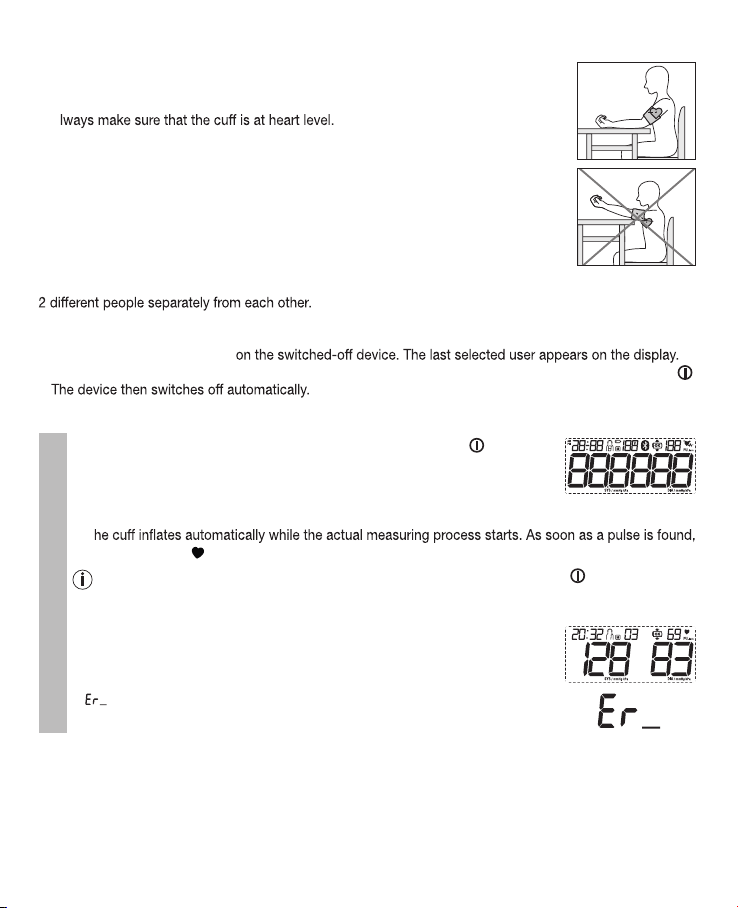
The blood pressure monitor is intended for the fully automatic, non-invasive measurement of arterial blood
pressure and pulse values on the upper arm.
Target group
It is designed for self-measurement by adults in the home environment and is suitable for users whose upper
Indication/clinical benefits
The user can record their blood pressure and pulse values quickly and easily using the device. The recorded
values are classified according to internationally applicable guidelines and evaluated graphically. Furthermore,
the device can detect any irregular heart beats that occur during measurement and inform the user via a
symbol in the display. The device saves the recorded measurements and can also output average values of
previous measurements. The recorded data can provide healthcare service providers with support during the
diagnosis and treatment of blood pressure problems, and therefore plays a part in the long-term monitoring
of the user’s health.
4. Warnings and safety notes
Contraindications
• D
• This device is not intended to be used as an Ambulatory Blood Pressure Monitor. Please do not apply the cuff for a long period of time
o not use the blood pressure monitor on newborns, children or pets.
• P eople with restricted physical, sensory or mental skills should be supervised by a person responsible for
their safety and receive instructions from this person on how to use the device.
• If you have any of the following conditions, it is essential you consult your doctor before using the device:
cardiac arrhythmia, circulatory problems, diabetes, pregnancy, pre-eclampsia, hypotension, chills, shaking.
• P eople with pacemakers or other electrical implants should consult their doctor before using the device.
• T he blood pressure monitor must not be used in connection with a high-frequency surgical unit.
• D
• D
• M -
ment, e.g. intravascular access or intravascular therapy, or an arteriovenous (AV) shunt.
General warnings
• T he measured values taken by you are for your information only – they are no substitute for a medical exam -
ination. Discuss the measured values with your doctor and never make your own medical decisions based
on them (e.g. regarding dosages of medicines).
• T he device is only intended for the purpose described in these instructions for use. The manufacturer is not
liable for damage resulting from improper or incorrect use.
• U
• The PATIENT is an intended OPERATOR
sing the blood pressure monitor outside your home environment or whilst on the move (e.g. whilst travelling
in a car, ambulance or helicopter, or whilst undertaking physical activity such as playing sport) can influence
the measurement accuracy and cause incorrect measurements.
• C
accuracy.