Bowa ERGO 310D User manual

MN031-600-S2
Instructions for use
en


BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
1
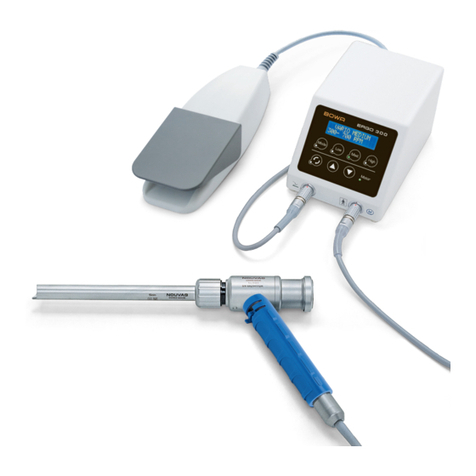
Legend
Knife
Jaws
Shaft
Rotation wheel
Active electrodes
HF cable with COMFORT plug
Handle with operating lever
Trigger
Finger switch

BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
2
1 Intended use
1.1 Purpose:
The ERGO 310D is an instrument for vessel sealing arterial and
venous blood vessels and vascularized tissue in laparoscopic
and open surgery procedures in many different surgical
disciplines (including but not restricted to general surgery,
gynecology, urology, thoracic surgery, etc.).
The instrument can also be used for cutting through tissue.
The instrument is intended for use with the bipolar vessel
sealing mode of BOWA ARC generators.
1.2 Contraindications:
Vessel sealing instruments must not be used if, in the opinion of
an experienced physician or according to current medical
literature, their use would put the patient at risk, for example,
due to the patient’s general condition or if there are other
contraindications.

BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
3
WARNING
Risk of Patient injury
Do not use with vessels larger than 7.0 mm in
diameter.
The reliability of sealing must be assessed by the
attending physician according to the nature of the
vascularised tissue and the vessel pathology
(arteriosclerosis, aneurysms, vascularisation, etc.).
Do not use on the heart, on the central circulatory
system or on the central nervous system.
Do not use for contraceptive coagulation of the
Fallopian tubes.
Do not use vessel sealing for tissue groups with
unknown content.
Proceed cautiously when performing sealing in the
vicinity of sensitive structures, such as nerves or
ureter.
Avoid grasping too much tissue for sealing – the jaws
should not be full.

BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
4
2 General safety instructions
The HF device may be used only by trained medical staff. The
surgeon and medical staff must be trained in the fundamental
principles, rules for use and risks of HF surgery and must be
familiar with them.
Read the operating manual carefully and thoroughly before
using the device!
Use aseptic techniques when removing the product from
the packaging.
Do not use the product if the packaging is opened or
damaged, the product will no longer be sterile. Do not re-
sterilize!
The product is intended for single use and may not be
resterilized. While autoclaving, the instrument gets
thermally damaged, deformed so that it loses its function.
Do not use the product if its expiry date has elapsed.
Do not use defective instruments.
All serious incidents arising in connection with the product
must be reported to the manufacturer and the competent
authority of the member state in which the user is
established.

BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
5
2.1 HF device
Use only approved HF devices and programs (see section
8, page 16).
Observe the operating instructions of the HF device and
the general instructions for electrosurgical operations!
The “BF”/”CF” applied part of the HF unit used is extended
by the instrument connected thereto.
Improper use of HF current can lead to endogenous and
exogenous burns and explosions:
Avoid direct skin contact with HF cables.
Avoid contact with flammable gases and liquids.
2.2 HF cable
Improper use of HF cables can lead to patient injuries:
Never lay the HF cable on the patient’s skin.
When plugging or unplugging the HF cable, always grasp
the connector directly.
Use only HF cables that are in perfect condition. Never use
a defective HF cable.
The HF cable may cause interference to imagery on monitors:
Never route the HF cable alongside a camera cable.
Do not lay the HF cable in loops.
Consult the operating manuals of the BOWA HF
generators for additional information on interference with
other devices.
2.3 Active electrodes and instrument
Defective or worn electrodes can cause burns to the patient:
Never use or repair worn or defective electrode surfaces.
Dispose of them.
Hot electrode surfaces may cause patient injuries:
Maintain sufficient distance between the tips of the
instrument and sensitive tissue structures, such as the
pancreas or intestine.
Ensure that hot instruments are not used for preparation.

BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
6
Inadvertent activation of the ligation instrument may cause
patient injuries:
Do not lay the ligation instrument on the patient.
Dirty electrodes may cause a short circuit, thereby resulting in
functional failure of the ligation instrument.
Clean the jaw electrodes regularly with a moist cloth.
2.4 EMC Information
Medical electrical devices are subject to special EMC
precautions, so please observe the following directions.
BOWA accessories are intended only for connection to HF
devices specified by BOWA.
Use of the accessories on medical devices from
manufacturers other than those described may lead to
elevated emissions or reduced interference immunity.
BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
7
2.5 Personal safety instructions
Incorrect HF generator configuration settings and limited
visibility can lead to patient injuries:
Carry out operations only with adequate visibility.
Use only the ligation program of BOWA ARC generators
with bipolar ligation current.
Prepare the tissue to be sealed so it is as free as possible
in order to avoid unintentional clamping.
Skin-to-skin contact (e.g. between the arms and body of
the patient) should be avoided, e.g. by placing dry
compresses between possible contact points.
2.6 Patients with pacemakers
Malfunctions or destruction of the pacemaker can endanger the
life of the patient or result in irreversible injuries to the patient:
Never perform ambulant operations on patients with
pacemakers.
In cases of patients with pacemakers, consult the
cardiologist before carrying out HF surgery.
NOTE
Combining medical devices is only safe if
the relevant instructions for use permit the desired
combination or
the purpose and the interface specification of the
products used in combination allow this.
The instructions for use and the interface specification
of the medical devices used in combination must be
strictly observed.

BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
8
Set the demand pacemaker to a fixed frequency.
Ensure that the pacemaker does not come into contact
with the HF electrode.
Keep a fully operational defibrillator within reach
Carry out a postoperative pacemaker check.

BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
9
3 Functionality
In bipolar HF surgery, tissue coagulation is achieved by
applying a high-frequency AC current, which generates heat.
The ERGO 310D ligation instruments are surgical invasive
instruments for use in laparoscopic or open surgery.
They are used through surgically generated access openings in
conjunction with products for endoscopic use, such as trocars
and optics.
The active electrodes (branches) are the non-insulated areas of
the jaws.
The HF current flows from one branch of the instrument through
the biological tissue to the other branch to produce the desired
localized coagulation effect.
With this method, sealing of a vessel or tissue segment carrying
blood is achieved by HF current in combination with
supplementary pressure.
The sealed location is hemostatically tight with respect to
systolic blood pressure and permanently closed.
The integrated cutting function allows the tissue under
treatment to be separated immediately after sealing without first
changing the instruments.
The jaws can be opened, closed and locked by actuating the
handle.
The jaw insert can be rotated by 220° using the rotation wheel
on the handle.
With the locking mechanism on the handle, a reproducible
mechanical pressure on the electrode tips is generated while
the handle is closed.
BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
10
4 Operation
4.1 Before use
1. Switch on the HF device.
2. Remove the product from the sterile packaging
3. Connect the HF cable to a bipolar socket of the HF device
4. The output power and mode is automatically set on the
BOWA ARC generator with the Plug’n Cut COMFORT
instrument detection.
5. Perform a thorough visual inspection and functional test
before using the ligation instrument.
4.2 Inspection
1. Check if the rotation wheel can be turned easily until the
stop is reached.
2. Check the jaws for proper opening and closing using the
lever
3. Lock the handle by pressing the lever down.
4. Pull the trigger to check for ease of motion.
5. Check the finger switch for proper activation.
The activation signal sounds while the finger switch is
being pushed.
WARNING
Risk of patient injury!
Use only intact products.
Risk of patient injuries from the combustion or
explosion of flammable liquids and gases!
Avoid contact with flammable gases and liquids, such
as skin cleaners, disinfectants and anaesthetic gases

BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
11
4.3 During the operation
WARNING
Risk of patient injury due to limited visibility!
Carry out operations only with adequate visibility.
Risk of patient injury due to hot electrode surfaces and
vapor emission!
Maintain sufficient distance between the tips of the
instrument and sensitive tissue structures, such as the
pancreas or intestine.
Ensure that hot ligation instruments are not used for
preparation.
Do not lay the ligation instrument on the patient.
Risk of patient injury due to inadvertent activation of
the ligation instrument!
Do not switch on the HF current before the active
electrodes are in contact with the tissue to be
coagulated.
Dirty electrodes may lead to functional failure of the
ligation instrument!
Clean the electrodes of the jaws regularly with a moist
cloth.
Risk of injury due to sharp blade
Do not activate the trigger of the blade during cleaning
of the electrodes.
Inserting the instrument
1. Close the electrodes using the handle.
2. Insert the instrument in the trocar sleeve.
BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
12
WARNING
Risk of patient injury due to inadvertent activation
of the vessel sealing instrument
Never use the AUTOSTART function.
Do not switch on the HF current before the active
electrodes are in contact with the tissue to be
coagulated and the handle is latched.
Accidental activation of the vessel sealing instrument
may cause injury to the patient
.
3. Place the jaw insert on the operation site.
4. Turn the rotation wheel to set the angle of the jaw insert.
5. Place the tissue to be sealed between the electrodes of the
jaws.
6. Close the jaws to grasp the tissue. Do not grasp too much
tissue.
The tissue is grasped.
7. Lock the handle.
The tissue is clamped.
8. Activate the HF current for coagulation using the finger
switch on the handle or footswitch:
•A continuous acoustic signal sounds during the
entire sealing process to indicate that power is
being supplied.
•An alternating acoustic signal indicates the end
of the sealing process.
•If there is a short-circuit in the area of the
sealing instrument or if the sealing instrument is not
in contact with tissue during activation, a warning will
be displayed on the HF device.
Please check the instrument and reactivate.

BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
13
WARNING
Incomplete sealing
Avoid contact with metallic objects (clips, stents, etc.)
in the area of the active electrode surfaces. They can
affect energy output and lead to undesired effects.
9. Release the finger switch or footswitch.
10. Press the handle further down to release the lock and to
open the jaws.
The tissue is coagulated.
Cutting the tissue
The tissue is grasped by the jaws and is coagulated.
WARNING
Strong bleeding may occur if the grasped tissue is cut
before coagulation or ligation!
Before cutting, ensure that the tissue has been reliably
sealed.
Cut only in the sealed area.
1. To cut, pull the trigger and then release it.
The tissue is cut in the centre of the sealed area.
BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
14
4.4 Withdrawal
WARNING
Risk of patient injury due to damaged or broken-off
parts!
Check the instrument after each withdrawal. All parts
must be present.
1. Close the jaws.
2. Withdraw the ligation instrument from the trocar sleeve.
4.5 After use
Discard the instrument after use. Do not resterilize.
5 Disposal
DANGER
Infection hazard!
To avoid spreading germs and
infections, sterilize the
instrument before it leaves the hospital or surgical
practice.
Always dispose of medical products, packaging materials and
accessories in accordance with applicable national regulations
and statutes.
BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
15
6 Storage
1. Store the ligation instrument in a location where it is
protected against:
•strong mechanical stresses such as shocks, falling
or blows;
•direct exposure to sunlight;
•X-ray radiation.
2. Store the ligation instrument in a dry place at room
temperature.
The shipping box is not intended for storing the device.
7 Transport
1. When transporting the instrument, make sure that it is protected
from:
•severe mechanical effects, such as shock, dropping or
impact,
•direct sunlight,
•X-rays,
•heat sources.
2. The instrument must be dry during transport.

BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
16
8 Technical specifications
Technical specifications
REF 775-000
Max. voltage 200 Vp sinusoidal
Approved
HF device BOWA ARC generators with vessel sealing
software:
• ARC 350 - REF 900-351
• ARC 400 - REF 900-400
Approved
programs LIGATION, ARCSeal
BOWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
17
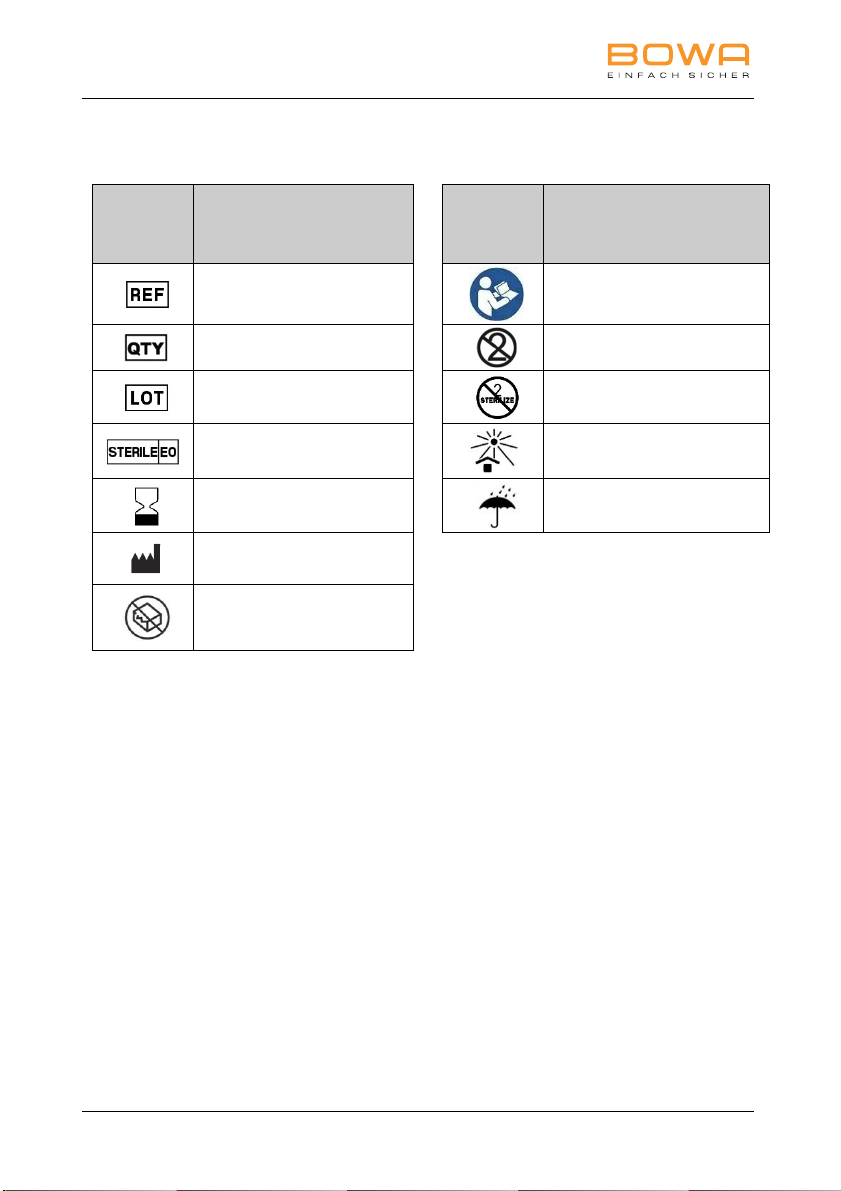
9 Symbols on the packaging
Icon /
Labeling Meaning Icon /
Labeling Meaning
Reference number
Follow the instructions
for use
Quantity
Single use only
Batch code
Do not resterilize
Sterilized with ethylene
oxide
Keep away from
sunlight
Expiry date
Keep dry
Manufacturer
Do not use if the
packaging is damaged

0123
B
OWA-IFU-MN031-600-ERGO310D-S2-EN-20190116
Printed in Germany │Subject to technical and design changes
Copyright by BOWA
-
electronic, Gomaringen
│Germany
BOWA-electronic GmbH & Co. KG
Heinrich-Hertz Strasse 4–10
D-72810 Gomaringen │Germany
Phone: +49 (0) 7072-6002-0
Fax: +49 (0) 7072-6002-33
info@bowa-medical.com
www.bowa-medical.com
CE Marked according to
Medical Device 93/42/EWG
Other manuals for ERGO 310D
2
Table of contents
Other Bowa Medical Equipment manuals

Bowa
Bowa TissueSeal PLUS COMFORT Series User manual

Bowa
Bowa ERGO 315R User manual

Bowa
Bowa ARC 400 User manual

Bowa
Bowa 901-011 User manual

Bowa
Bowa ERGO 310D User manual

Bowa
Bowa 901-011 User manual

Bowa
Bowa TissueSeal PLUS COMFORT User manual

Bowa
Bowa ARC 350 User manual

Bowa
Bowa Lotus User manual

Bowa
Bowa ERGO 315R User manual
Popular Medical Equipment manuals by other brands
Nasco
Nasco Life/form LF00901U instruction manual

Orliman
Orliman Pediatric OP1197 Use and maintenance instructions

Olympus
Olympus EVIS EXERA III instructions

UV MEDICO
UV MEDICO UV222 Technical and installation manual

burmeier
burmeier Regia instruction manual

Thoratec
Thoratec HEARTMATE II Instructions for use