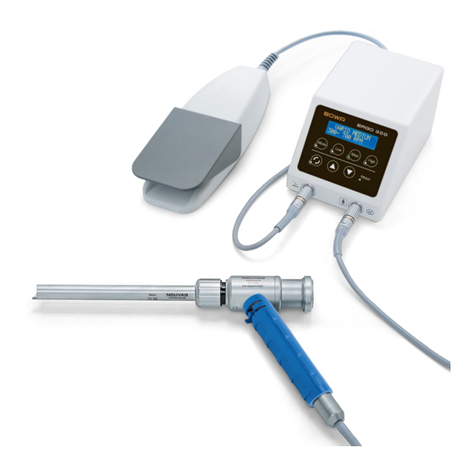
Bowa ERGO 315R User manual

MN031-554-S3 en
Instructions for use

Key
2 BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219
Key
Blade with blade holder
Blade rod
Push rod
Jaw
Electrodes
Teeth
Mounting
marking
Slotted ring
for blade rod
latching
Blade mount
Blade
Grip area
Blade holder
Spring latch
Cleaning tip
Mounting aid

Key
BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219 3
Handle
Cable with handswitch
Handswitch
Connector for
BOWA ARC
generator
Mounting
marking
Locking mechanism
Rotation wheel
Push buttons
Blade trigger
Jaw actuation lever
Jaw holder

Key
4 BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219
Cleaning adapter
Cleaning brush
Reprocessing basket & lid
Cleaning adapter jaw /
handle
Cleaning adapter
push rod

Contents
BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219 5
Contents
Key 2
Contents.....................................................................................5
1. Using these instructions for use.....................................7
1.1. Scope of validity.................................................................7
1.2. Symbols and notation.........................................................8
2. Intended Purpose.............................................................9
2.1. Indications..........................................................................9
2.2. Contraindications................................................................9
3. Safety instructions...........................................................9
3.1. Device-related....................................................................9
3.2. Use-related.......................................................................10
3.2.1.Patients with pacemakers.................................................12
3.3. EMC information...............................................................12
4. Mode of operation..........................................................13
5. Assembly........................................................................14
6. Operation........................................................................24
6.1. Before use........................................................................24
6.2. Function test in the operating theatre...............................25
6.3. During surgery..................................................................25
6.4. Removal...........................................................................27
6.5. After use...........................................................................27
7. Dismantling.....................................................................28
7.1. Dismantling the vessel sealing instrument........................28
7.2. Deviating from the assembly ............................................29
7.3. Removing the blade .........................................................31

Contents
6 BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219
8. Reprocessing .................................................................32
8.1. Dismantling ......................................................................33
8.2. Soaking............................................................................34
8.3. Manual removal of contamination.....................................35
8.4. Pretreatment in an ultrasonic bath....................................35
8.5. Machine-based reprocessing in a CDM............................36
8.5.1.Preparation for machine-based reprocessing –cleaning
adapter.............................................................................36
8.5.2.Placing the parts in the reprocessing basket ....................38
8.6. Inspection.........................................................................41
8.7. Packing............................................................................42
8.8. Autoclaving.......................................................................43
8.9. Environmental conditions .................................................44
8.9.1.Storage and transport.......................................................44
8.9.2.Operation .........................................................................44
9. Technical data ................................................................45
10. Disposal..........................................................................45
11. Symbols on packaging ..................................................46

1 Using these instructions for use
BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219 7
1. Using these instructions for use
These instructions for use are part of the device. BOWA-electronic GmbH
& Co. KG assumes no liability and provides no warranty whatsoever for any
damage or consequential damage arising from non-compliance with these
instructions for use.
Read the instructions for use, in particular the section on safety (see section
Safety instructions, page 9) carefully and thoroughly before use.
Store the instructions for use in a safe place throughout the service life
of the device.
Keep the instructions for use accessible to operating theatre
personnel.
Give the instructions for use to each successive owner or user of this
device.
Always update the instructions for use whenever you receive
additional information from the manufacturer.
1.1. Scope of validity
These instructions for use apply only to the parts of the ERGO 315R
instrument listed below.
REF
Designation
770-510
ERGO 315R Handle
770-522
ERGO 315R Jaw 275 mm
770-523
ERGO 315R Jaw 360 mm
770-532
ERGO 315R Push rod 275 mm
770-533
ERGO 315R Push rod 360 mm
770-542
ERGO 315R Blade rod 275 mm
770-543
ERGO 315R Blade rod 360 mm
770-998
ERGO 315R Blade sterile (10 pcs.)
358-245
ERGO 315R Cable with handswitch
723-050
ERGO 315R Cleaning adapter set
773-982
ERGO 315R Reprocessing basket
773-983
ERGO 315R Lid for basket
723-000
Cleaning brush set

1 Using these instructions for use
8 BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219
1.2. Symbols and notation
Structure of warning instructions
SIGNAL WORD
Type, source and consequences of the hazard
(personal injuries)!
Measures for avoiding the hazard.
Hazard levels of warning instructions
Symbol
Hazard level
Probability
of occurrence
Consequences
of non-compliance
DANGER
Immediate risk
Death or serious injuries
WARNING
Possible risk
Death or serious injuries
CAUTION
Possible risk
Minor injuries
NOTE
Possible risk
Property damage
Tips
Tips to make your work easier or supplementary explanatory
information for a procedure.
Other symbols and notation
Symbol / notation
Meaning
Prerequisite for an activity
Activity with one step
1.
2.
Activity with several steps in strict
sequence
Result of preceding activity
•
List (first level)
•
List (second level)
Emphasis
Emphasis
..., see section xxx, page xxx
Cross-reference

2 Intended Purpose
BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219 9
2. Intended Purpose
Electrosurgical equipment for cutting and coagulation of tissue.
2.1. Indications
The vessel sealing/ligation instrument is intended to be used to seal arterial
and venous blood vessels and vascular tissue structures in laparoscopic
and open surgical procedures in various surgical disciplines (including but
not limited to general surgery, gynaecology, urology, thoracic surgery and
others). The instrument can also be used to cut tissue.
2.2. Contraindications
Vessel sealing/ligation instruments must not be used in direct contact with
the heart, central nervous system or central circulatory system.
Do not use vessel sealing/ligation instruments if their surgical techniques
are contraindicated.
Do not use vessel sealing/ligation instruments if, in the opinion of an
experienced physician or according to current specialist literature, such use
would endanger the patient, for example due to the patient’s general
condition, or if other contraindications are present.
3. Safety instructions
3.1. Device-related
•The instrument may only be used by trained medical staff. The surgeon
and the medical specialist personnel must be trained in, and be familiar
with, the fundamentals, codes of practice and risks of HF surgery.
Read the instructions for use carefully and thoroughly before using
the device
All serious incidents occurring in connection with the device must
be reported to the manufacturer and to the competent authority of
the country in which the user is established.
•The devices are supplied in non-sterile condition and must be cleaned
and sterilised before use.
•Do not reprocess the replaceable blade and blade holder.
Risk of injury from sharp blade
Discard and replace used blades and blade holders.
•The device must not be operated in AUTOSTART mode.
•As part of your responsibility for the sterility of the instruments used,
please note:

3 Safety instructions
10 BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219
Clean and sterilise the ERGO 315R handle and the associated
electrode before each use.
Use only cleaning, disinfection and sterilisation methods that have
been validated for the specific devices and products concerned.
Comply with the validated parameters for each cycle.
Observe the applicable legal regulations in your country and the
hygiene regulations of the hospital.
To clean the instrument correctly, it is essential to remove the
replaceable blade.
Do not carry out gas sterilisation and hot air sterilisation, they can
damage the product.
•To avoid combustion (thermal overload), observe the following points:
Make sure that all electrical plug connections are correctly
connected.
Do not stress the products beyond their mechanical limits (e.g. by
excessive bending, buckling or crushing).
The lowest maximum voltage of the product must not be exceeded.
•In order to avoid the risk of injuries and electric shocks for the patient
and the operating personnel, ensure that the power supply is switched
off before connecting or removing the HF connection cable and
accessories to or from the electrosurgical device.
3.2. Use-related
•Only the Ligation and ARC SEAL modes may be used with the
BOWA ARC generators.
•The electrode should only be activated with suitable visual control.
•Do not use the product on vessels with a diameter greater than 7.0 mm.
•Do not use vessel sealing for tissue groups with unknown content.
•Proceed cautiously and maintain distance when performing sealing in
the vicinity of sensitive structures, such as nerves or ureter
•Do not use too much tissue for sealing. The jaw must not be too full.
•The reliability of sealing must be assessed by the attending physician
according to the nature of the vascularised tissue and the vessel
pathology (arteriosclerosis, aneurysms, vascularisation, etc.).

3 Safety instructions
BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219 11
•Improper use of HF current can lead to burns and explosions:
Carry out electrosurgery only with insufflation of non-flammable
gases (CO2).
Avoid direct skin contact with HF cables.
Avoid contact with flammable gases and liquids.
•Improper use of the product can lead to patient injuries:
Avoid skin-to-skin contact between the HF cable and the patient.
When plugging or unplugging the HF cable, always grasp the
connector directly.
Do not place the handle on the patient as personal injury
may result should accidental HF activation occur.
Avoid contact with metallic objects (clips, stents, etc.) in the area of
the active electrode surfaces. They can affect energy output and
lead to undesired effects.
•The electrode tip may still be hot enough to cause burns immediately
after deactivating the HF power.
•The HF cable may cause interference to imagery on monitors:
Never route the HF cable alongside a camera cable.
Do not lay the HF cable in loops.
Consult the instructions for use of the BOWA HF generators for
additional information on interference with other devices.
•Do not repair or service defective devices:
Check the device for damage after reprocessing and before use.
CAUTION: Visual inspection on its own is not sufficient for ensuring
the integrity of the insulation.
Discard or replace defective devices.
•Observe the instructions for use of the HF device and the general
instructions for electrosurgical operations!
•The "BF" / "CF" application part of the HF device used is extended by
the instrument connected to it.
3 Safety instructions
12 BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219
3.2.1. Patients with pacemakers
Malfunction or destruction of the pacemaker can endanger the life of the
patient or result in irreversible injuries to the patient.
Carry out a postoperative pacemaker check.
3.3. EMC information
Medical electrical devices are subject to special EMC precautions,
so please observe the following directions.
The BOWA accessory is only intended to be connected to BOWA specified
HF devices.
Using the accessory with medical devices from other manufacturers
can result in higher emission levels or reduced interference immunity.
Never perform outpatient operations on patients with pacemakers.
In cases of patients with pacemakers, consult the cardiologist before
carrying out HF surgery.
Set the demand pacemaker to a fixed frequency.
Ensure that the pacemaker does not come into contact with the
HF electrode.
Keep a fully operational defibrillator within reach
NOTE
Combining medical devices is only safe if
the desired combination is allowed by the instructions for
use, and
the purpose and the interface specification of the devices
used in the combination allow it.
Strict attention must be given to the instructions for use and
interface specifications of medical devices used in
combination.

4 Mode of operation
BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219 13
4. Mode of operation
In bipolar HF surgery, tissue coagulation is achieved by applying a high-
frequency AC current, which generates heat.
The ERGO 315R vessel sealing instruments are invasive surgical
instruments for use in laparoscopic or open surgery.
They are used through surgically produced access openings in conjunction
with devices for endoscopic use, such as trocars and optics.
The active electrodes are the non-insulated areas of the jaws. The HF
current flows from one electrode of the instrument through the biological
tissue to the other electrode to produce the desired localised tissue effect.
With this method, sealing of a vessel or tissue segment carrying blood is
achieved by HF current in combination with supplementary pressure. The
sealed location is haemostatically tight with respect to systolic blood
pressure and permanently closed.
The instrument can be used for vessels with diameters up to 7 mm.
The integrated cutting function of the ERGO 315R allows the tissue to be
cut under treatment immediately after sealing without first changing
instruments.
The electrodes can be opened, closed and latched by actuating the handle.
A ratchet mechanism in the handle generates a reproducible mechanical
pressure on the electrode tips when the handle is closed. The jaw can be
rotated and positioned by a rotation wheel on the handle.
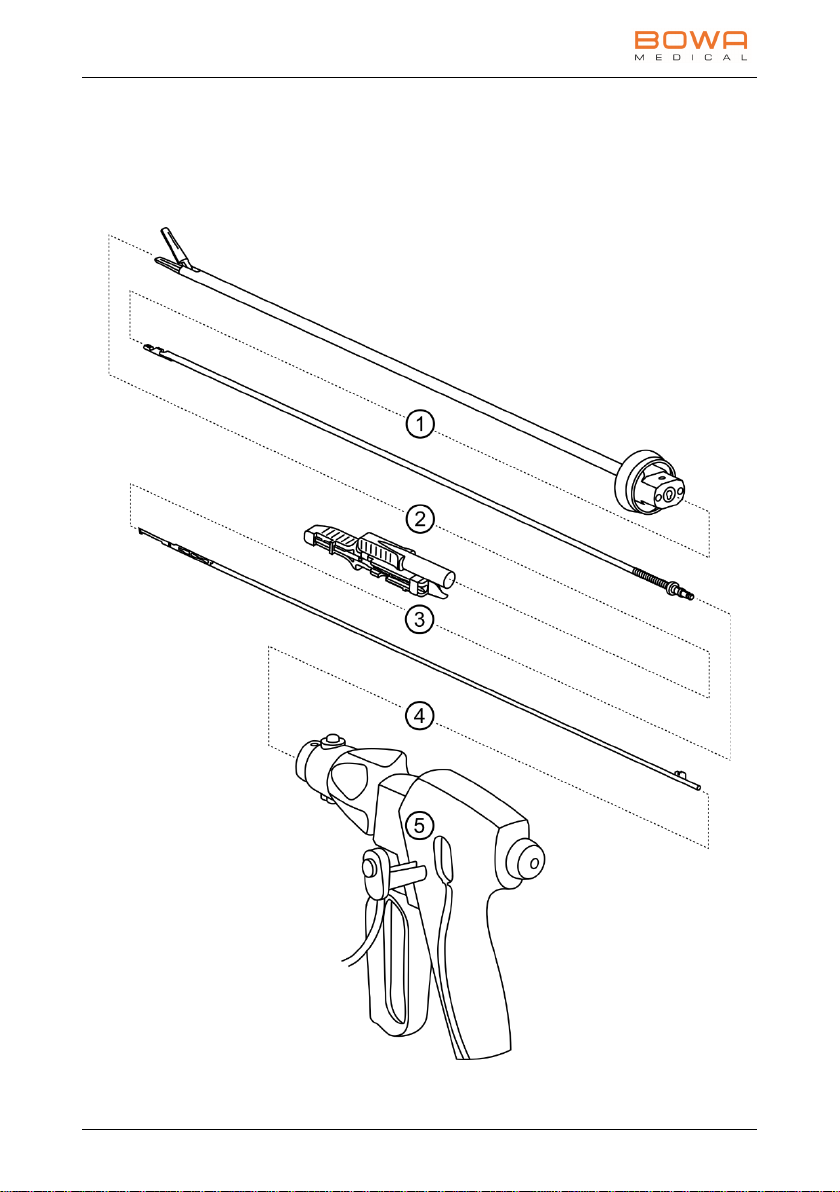
5 Assembly
14 BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219
5. Assembly
Assembling the vessel sealing instrument
Assemble the vessel sealing instrument in the sequence shown below:
Inserting the push rod into the jaw

5 Assembly
BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219 15
Insert the push rod into the completely opened jaw with the electrodes
fully opened.
Align the teeth of the push rod with the orange marking on the jaw.
Attaching the mounting aid

5 Assembly
16 BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219
The blade with blade holder and mounting aid are supplied
sterilised.
1. Remove the blade with blade holder and mounting aid from the
sterile package.
2. Insert the jaw with mounted push rod and closed electrodes into
the mounting aid on the blade holder.

5 Assembly
BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219 17
3. Separate the blade holder from the mounting aid by pressing
down the blade holder in the grip area.

5 Assembly
18 BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219
Mounting the blade with the blade holder
WARNING
Risk of injury from sharp blade!
To avoid punctures and lacerations, always use the blade
holder for blade assembly.
To mount the blade, grasp the blade holder in the grip area.
Position the blade mount on the blade as indicated by the
markings on the blade holder and slide the blade rod in the
direction indicated by the arrow until the blade audibly engages.
Lift the blade rod with the mounted blade slightly and pull it out
of the blade holder.
Avoid bending the blade in the process.

5 Assembly
BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219 19
1. Visually check the blade for correct assembly.
The blade holder has a cleaning tip for cleaning the blade
groove. Keep it until the end of the surgery to clean the groove
if necessary.

5 Assembly
20 BOWA-IFU-MN031-554-ERGO315R-S3-EN-20210219
Inserting the blade rod into the push rod
Insert the blade rod with the assembled blade into the push rod.
Align the spring latch of the blade rod with the slotted ring of the
push rod and insert the blade rod until it audibly engages.
Other manuals for ERGO 315R
2
Table of contents
Other Bowa Medical Equipment manuals

Bowa
Bowa ARC 350 User manual

Bowa
Bowa 901-011 User manual

Bowa
Bowa TissueSeal PLUS COMFORT User manual

Bowa
Bowa ARC 100 User manual

Bowa
Bowa ARC 400 User manual

Bowa
Bowa ErgoLAP BIPOLAR User manual

Bowa
Bowa TissueSeal PLUS COMFORT Series User manual

Bowa
Bowa ERGOeco User manual

Bowa
Bowa ARC PLUS User manual

Bowa
Bowa ERGOact User manual
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual