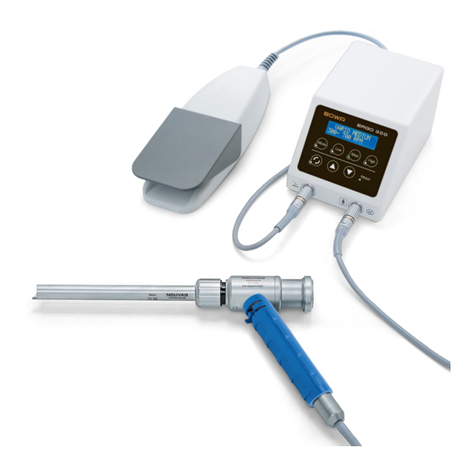
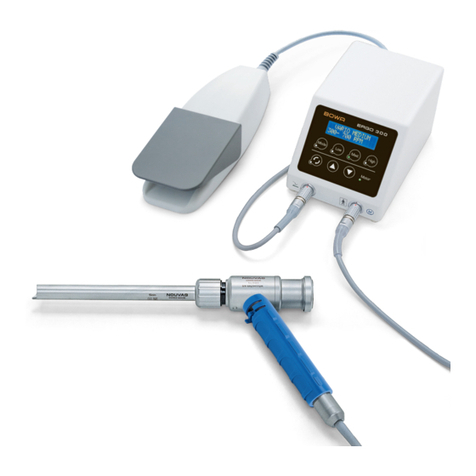
BOWA-IFU-12578-ERGOACT-S0-EN-20210715 Instructions for Use ERGOact 3
Key ...................................................................................................... 1
1 Applying the instructions for use ...................................................... 4
1.1 Scope of validity .....................................................................................................4
1.2 Symbols and markings ...........................................................................................4
2 Purpose ............................................................................................. 6
2.1 Indications ..............................................................................................................6
2.2 Contraindications ...................................................................................................6
3 Safety instructions ............................................................................ 7
3.1 Device-related ........................................................................................................7
3.2 Use-related .............................................................................................................8
3.2.1 Patients with pacemakers ...............................................................................9
3.3 EMC information ....................................................................................................10
4 Mode of operation ............................................................................ 11
5 Procedure during use ....................................................................... 12
5.1 Connection combinations .......................................................................................12
6 Assembly .......................................................................................... 13
7 Operation ......................................................................................... 14
7.1 Before use ..............................................................................................................14
7.2 Function test in the operating theatre ...................................................................14
7.3 During the operation ..............................................................................................15
7.4 Removal ..................................................................................................................16
7.5 After use .................................................................................................................17
7.5.1 Spare parts .....................................................................................................17
8 Dismantling ...................................................................................... 18
9 Pre-conditioning ............................................................................... 19
9.1 General information ...............................................................................................19
9.2 Device-specific information ....................................................................................19
9.3 Inspection ...............................................................................................................21
9.4 Packaging ...............................................................................................................22
10 Environmental conditions ............................................................... 22
10.1 Storage and transport ..........................................................................................22
11 Technical data ................................................................................. 23
12 Disposal .......................................................................................... 23
13 Symbols on packaging .................................................................... 24