Bowa TissueSeal PLUS COMFORT Series User manual

EN
Operating Manual
TissueSeal®PLUS COMFORT
TissueSeal®COMFORT


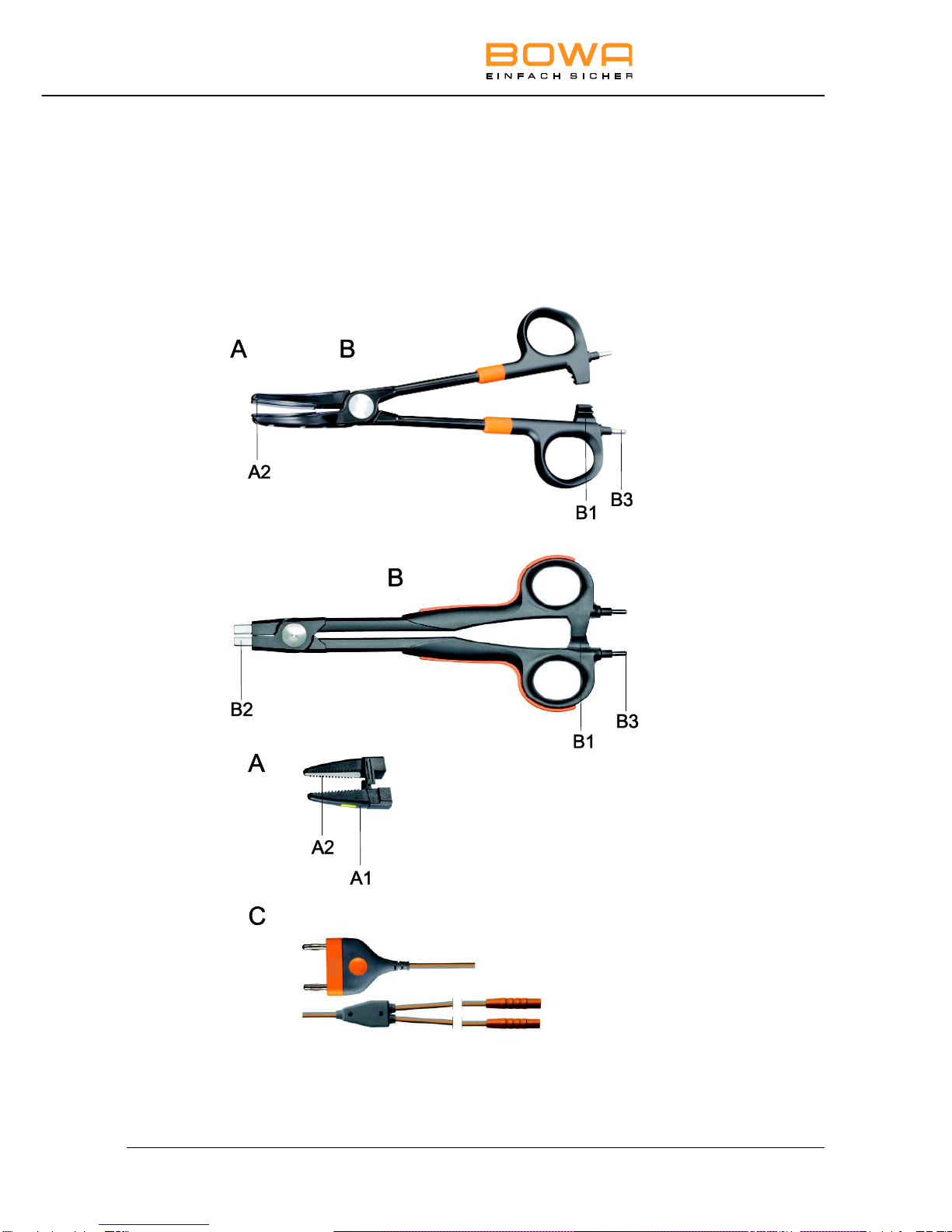
Legend
MN031-543-S0 EN TissueSeal / TissueSeal PLUS COMFORT 3
Legend
4 TissueSeal / TissueSeal PLUS COMFORT MN031-543-S0 EN

Legend
MN031-543-S0 EN TissueSeal / TissueSeal PLUS COMFORT 5
Legend
A Jaws B Handle
A1 Disposable electrode tip B1 Ratschet
A2 Electrode surfaces B2 Jaws holder
B3 HF cable
B3
B1
A
B
A2
B
A
B2
B1
B3
A2
A1

Contents
6 TissueSeal / TissueSeal PLUS COMFORT MN031-543-S0 EN
Contents
Legend.........................................................................................................5
Contents......................................................................................................6
1. Using this operating manual..............................................................8
1.1. Validity......................................................................................8
1.2. Icons and labeling ....................................................................9
1.2.1 Structure of warning instructions....................................9
1.2.2 Risk levels in the warning instructions...........................9
1.2.3 Tips ................................................................................9
1.2.4 Other icons and labeling...................................................10
1.2.5 Symbols on packaging.................................................10
2. Safety .................................................................................................11
2.1 Intended use...........................................................................11
2.2 General safety instructions.....................................................12
2.2.1. HF device.....................................................................13
2.2.2. HF cable.......................................................................13
2.2.3. Active electrodes................................................................14
2.2.4. Repairs and servicing........................................................14
2.3 Personal safety instructions ...................................................15
2.3.1. Patients with pacemakers............................................15
3. Functionality......................................................................................16
4. Assembly ...........................................................................................17
4.1. Assembling the ligation instrument ........................................17
5. Operation ...........................................................................................17
5.1. Before use..............................................................................17
5.2. During the operation...............................................................18
5.3. Withdrawal..............................................................................20
5.4. After use.................................................................................21

Contents
MN031-543-S0 EN TissueSeal / TissueSeal PLUS COMFORT 7
6. Dismantling........................................................................................21
6.1. Dismantling the ligation instrument........................................21
7. Preparation ........................................................................................22
7.1. Soaking ..................................................................................23
7.2. Dismantling.............................................................................24
7.2.1. Pretreatment in an ultrasonic bath...............................24
7.3. Manual removal of contamination..........................................25
7.4. Automatic preparation in a CDM............................................26
7.5. Inspection...............................................................................28
7.6. Packing...................................................................................29
7.7. Autoclaving.............................................................................30
7.8. Storage...................................................................................31
7.9. Transport................................................................................32
7.10. Functional test in the operating room.....................................32
7.11. Recommended operating supplies.........................................32
8. Technical specifications ..................................................................33
8.1. TissueSeal / TissueSeal PLUS COMFORT...........................33
9. Disposal .............................................................................................34
10. System overview...............................................................................35
10.1. TissueSeal COMFORT ..........................................................35
10.2. TissueSeal PLUS COMFORT................................................36

1Using this operating manual
8 TissueSeal / TissueSeal PLUS COMFORT MN031-543-S0 EN
1. Using this operating manual
This operating manual is part of the device.
BOWA-electronic GmbH & Co. KG assume no liability nor provide any
warranty whatsoever for any damage or consequential damage arising
from non-compliance with this operating manual.
Read the operating manual, in particular the safety instructions
(see section 2, page 9)carefully and thoroughly before use.
Store the operating manual in a safe place throughout the service
life of the device.
Keep the operating manual accessible to operating room
personnel.
Give the operating manual to each successive owner and/or user
of this device.
Always update the operating manual whenever you receive
additional information from the manufacturer.
1.1. Validity
This operating manual applies only to the devices designated on the
title page.
1Using this operating manual
MN031-543-S0 EN TissueSeal / TissueSeal PLUS COMFORT 9
1.2. Icons and labeling
1.2.1 Structure of warning instructions
SIGNAL WORD
"Risk type, source and consequences there of"
(Personal injury)!
Measure for risk prevention.
1.2.2 Risk levels in the warning instructions
Symbol
Risk level
Probability of
occurrence
Consequences of
non-compliance
DANGER
Immediate risk
Death or serious
injuries
WARNING
Possible risk
Death or serious
injuries
CAUTION
Possible risk
Minor injuries
NOTE
Possible risk
Property damage
1.2.3 Tips
Tips to make your work easier or supplementary explanatory
information for a procedure.

1Using this operating manual
10 TissueSeal / TissueSeal PLUS COMFORT MN031-543-S0 EN
1.2.4 Other icons and labeling
Icon/Label
Meaning
Prerequisite for an activity
Activity with one step
1.
2.
3.
Activity with several steps in a binding
sequence
Result of preceding activity
List (first level)
List (second level)
Emphasis
Emphasis
..., see section xxx, page xxx
Cross reference
1.2.5 Symbols on packaging
Icon/ Labeling
Meaning
Manufacturer
Observe the operating instructions
2Safety
MN031-543-S0 EN TissueSeal / TissueSeal PLUS COMFORT 11
2. Safety
2.1 Intended use
The ligature instruments are intended to be used to seal arterial and
venous blood vessels and vascular tissue structures in open surgical
procedures in gynecology, urology, general surgery, and other
surgical disciplines with the aid of an HF current and mechanical
pressure.
In addition, the ligature instruments are suitable for conventional
bipolar coagulation.
The ligature instruments are intended to be used with the bipolar
“LIGATION” operating mode.
They are intended to be used in connection with the ligation program
of BOWA ARC generators.
Do not use the ligation instruments if, in the opinion of an experienced
physician or according to current professional literature, such use
would cause endangerment of the patient due, for example, to the
general condition of the patient, or if other contraindications are
present.
Do not use on the heart, the central circulation system or the central
nervous system.
The connection cable is permanently connected to the handle for
COMFORT items 760-216, 760-219, 760-223, 760-228, 760-319, 760-
323, 760-328.
Generators with Plug’Cut COMFORT detect BOWA COMFORT
instruments and automatically pre-select the respective parameters.
It is not necessary to use a neutral electrode with bipolar
ligation instruments.

2Safety
12 TissueSeal / TissueSeal PLUS COMFORT MN031-543-S0 EN
2.2 General safety instructions
The HF device may be used only by trained medical staff. The
surgeon and medical staff must be trained in the fundamental
principles, rules for use and risks of HF surgery and must be familiar
with them.
Read the operating manual carefully and thoroughly before using
the device!
In connection with your responsibility for the sterility of the ligation
instruments, observe the following when using them:
Clean and sterilize the ligation instrument before using it for the
first time. It is not sterile as delivered. With TissueSeal, the
disposable electrode tips are supplied in sterile condition.
Clean and sterilize the ligation instrument before each
subsequent use.
Use only cleaning, disinfection and sterilization methods that
have been adequately validated for the specific devices and
products concerned.
Comply with the validated parameters for each cycle.
Observe the applicable legal requirements in your country and
the hygiene regulations of the hospital.
In case of cleaning in an ultrasound bath or manual precleaning, there
is a risk of infection due to water spray and vapors:
Wear a face mask and protective clothing
Adequate ventilation is recommended.

2Safety
MN031-543-S0 EN TissueSeal / TissueSeal PLUS COMFORT 13
2.2.1. HF device
Use only approved HF devices and programs (see
section section 8, page 31).
Observe the operating instructions of the HF device and the
general instructions for electrosurgical operations!
Improper use of HF current can lead to endogenous and exogenous
burns and explosions:
Avoid direct skin contact with HF cables.
Avoid contact with flammable gases and liquids.
2.2.2. HF cable
Improper use of HF cables can lead to patient injuries:
Never lay the HF cable on the patient’s skin.
Connect the ligation instrument for coagulation before switching
on the HF generator.
When plugging or unplugging the HF cable, always grasp the
connector directly.
Use only HF cables that are in perfect condition. Never use a
defective HF cable.
The HF cable may cause interference to imagery on monitors:
Never route the HF cable alongside a camera cable.
Do not lay the HF cable in loops.
Consult the operating manuals of the BOWA HF generators for
additional information on interference with other devices.

2Safety
14 TissueSeal / TissueSeal PLUS COMFORT MN031-543-S0 EN
2.2.3. Active electrodes
Defective or worn electrodes can cause burns on patients:
Never use or repair worn or defective electrode surfaces.
Dispose of them.
Hot electrode surfaces may cause patient injuries:
Maintain sufficient distance between the tips of the instrument
and sensitive tissue structures, such as the pancreas or intestine.
Ensure that hot instruments are not used for preparation.
Inadvertent activation of the ligation instrument may cause patient
injuries:
Do not lay the ligation instrument on the patient.
Dirty electrodes may cause a short circuit, thereby resulting in
functional failure of the ligation instrument.
Clean the jaw electrodes regularly with a moist cloth.
2.2.4. Repairs and servicing
Do not repair or service defective devices:
Discard or replace defective devices.

2Safety
MN031-543-S0 EN TissueSeal / TissueSeal PLUS COMFORT 15
2.3 Personal safety instructions
Incorrect HF generator configuration settings and limited visibility can
lead to patient injuries:
Select the HF generator and the HF cable according to the
requirements of the ligation instrument.
Carry out operations only with adequate visibility.
Never use ligation instruments with the AUTOSTART function.
Use only the ligation program of Bowa ARC generators with
bipolar ligation current.
Prepare the tissue to be sealed so it is as free as possible in
order to avoid unintentional clamping.
2.3.1. Patients with pacemakers
Malfunctions or destruction of the pacemaker can endanger the life of
the patient or result in irreversible injuries to the patient:
Never perform ambulant operations on patients with pacemakers.
In cases of patients with pacemakers, consult the cardiologist
before carrying out HF surgery.
Set the demand pacemaker to a fixed frequency.
Ensure that the pacemaker does not come into contact with the
HF electrode.
Keep a fully operational defibrillator within reach
Carry out a postoperative pacemaker check.
Pos: 5 /679-BOWA/HF-Instrumente/Ligator "TissueSeal und TissueSeal
Plus"/03. Funktionsweise @ 5\mod_1291718350323_6.doc @ 46097 @ 1
3Functionality
16 TissueSeal / TissueSeal PLUS COMFORT MN031-543-S0 EN
3. Functionality
In bipolar HF surgery, tissue coagulation is achieved by applying a
high-frequency AC current, which generates heat.
The TissueSeal and TissueSeal PLUS ligation instruments are
invasive surgical instruments for use in open surgery.
The active electrodes (electrode surfaces) are the non-insulated areas
of the jaws.
The HF current flows from one electrode surface of the instrument
through the biological tissue to the other electrode surface and
produces the desired coagulation in a localized region.
With this method, sealing of a vessel or tissue segment carrying blood
is achieved by HF current in combination with supplementary
pressure.
The sealed location is hemostatically tight with respect to systolic
blood pressure and permanently closed.
760-216: Applies only to vessels up to 5 mm in diameter
760-219, 760-223, 760-228, 760-319, 760-323, 760-328:
Applies only to vessels up to 7 mm in diameter.
The jaws can be opened and closed by actuating the handle.
A ratchet mechanism in the handle provides reproducible mechanical
pressure on the electrode tips when the handle is closed.
Pos: 6 /679-BOWA/HF-Instrumente/Ligator "NightKNIFE"/04.Montage @
5\mod_1277985233074_6.doc @ 40636 @ 1
4Assembly
MN031-543-S0 EN TissueSeal / TissueSeal PLUS COMFORT 17
4. Assembly
WARNING
Risk of patient injury from non-sterile ligation
instruments!
The ligation instrument is not sterile as delivered. Clean
and sterilize the instrument before using it.
4.1. Assembling the ligation instrument
1. With TissueSeal: plug the disposable electrode tip A1 onto the
holder for jaw B2.
5. Operation
5.1. Before use
The ligation instrument is assembled (see section 4) and prepared (see
section 7, page 20).
WARNING
Risk of patient injury!
Use only approved BOWA ARC generators with ligation
capability (see section 8, page 31).
Use only suitable products and accessories as
described in the system overview.
Use only intact, sterilized products.
5Operation
18 TissueSeal / TissueSeal PLUS COMFORT MN031-543-S0 EN
WARNING
Risk of patient injuries from the combustion or
explosion of flammable liquids and gases!
Avoid contact with flammable gases and liquids, such
skin cleaners, disinfectants and anesthetic gases.
1. Connect the HF cable B3 to the HF device and switch on the HF
device.
2. Set the output power of the HF device.
3. Perform a thorough visual inspection and functional test each
time before using the ligation instrument (see section 7.5, page
26).
5.2. During the operation
WARNING
Risk of patient injury due to incorrect device settings
and limited visibility!
Set the output power of the HF device to the value
necessary for the operation.
Use only approved programs (see section 8, page 31).
Carry out operations only with adequate visibility.
5Operation
MN031-543-S0 EN TissueSeal / TissueSeal PLUS COMFORT 19
WARNING
Risk of patient injury due to hot electrode surfaces and
vapor emission!
Maintain sufficient distance between the tips of the
instrument and sensitive tissue structures, such as the
pancreas or intestine.
Ensure that hot ligation instruments are not used for
preparation.
Do not lay the ligation instrument on the patient.
Grasping, clamping and sealing tissue
WARNING
Risk of patient injury due to inadvertent activation of the
ligation instrument!
Never use the AUTOSTART function.
Do not switch on the HF current before the active
electrodes are in contact with the tissue to be
coagulated and the ratchet is closed.
1. Place the jaws Aon the operating site.
2. Place the tissue to be sealed between the electrodes of the jaws
A.
3. Close the jaws Ato grasp the tissue. Do not grasp too much
tissue.
The tissue is grasped.
4. Use the two-position ratchet of the handle Bto adjust the
pressure on the tissue to best match the amount of grasped
tissue.
The tissue is clamped.
5Operation
20 TissueSeal / TissueSeal PLUS COMFORT MN031-543-S0 EN
5. Using the foot switch of the HF device, activate the HF current for
coagulation:
A continuous acoustic signal sounds during the entire
sealing process to indicate that power is being supplied.
An alternating acoustic signal indicates the end of the
sealing process.
6. Release the foot switch to stop the supply of power.
7. Using the handle B, open the jaws A.
The tissue is coagulated.
Changing the output power of the HF device
Before increasing the output power of the HF device, check:
all HF cables and connectors are properly connected;
the ligation instrument is properly connected (see the
operating instructions of the HF device);
the foot switch works properly;
the insulation of the HF cable,
the active electrodes of the jaws Aare clean and not worn
out.
5.3. Withdrawal
WARNING
Risk of patient injury due to damaged or broken-off
parts!
Check the ligation instrument after each use. All parts
must be present.
This manual suits for next models
1
Table of contents
Other Bowa Medical Equipment manuals

Bowa
Bowa ErgoLAP BIPOLAR User manual
Bowa
Bowa ERGO 300 User manual

Bowa
Bowa 901-011 User manual

Bowa
Bowa SHE SHA User manual

Bowa
Bowa ARC 350 User manual

Bowa
Bowa ERGO 315R User manual

Bowa
Bowa TissueSeal PLUS COMFORT User manual

Bowa
Bowa ERGO 310D User manual

Bowa
Bowa ARC 100 User manual

Bowa
Bowa ERGOact User manual
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual

























