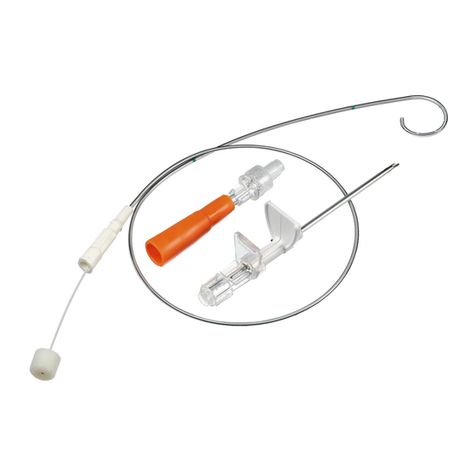
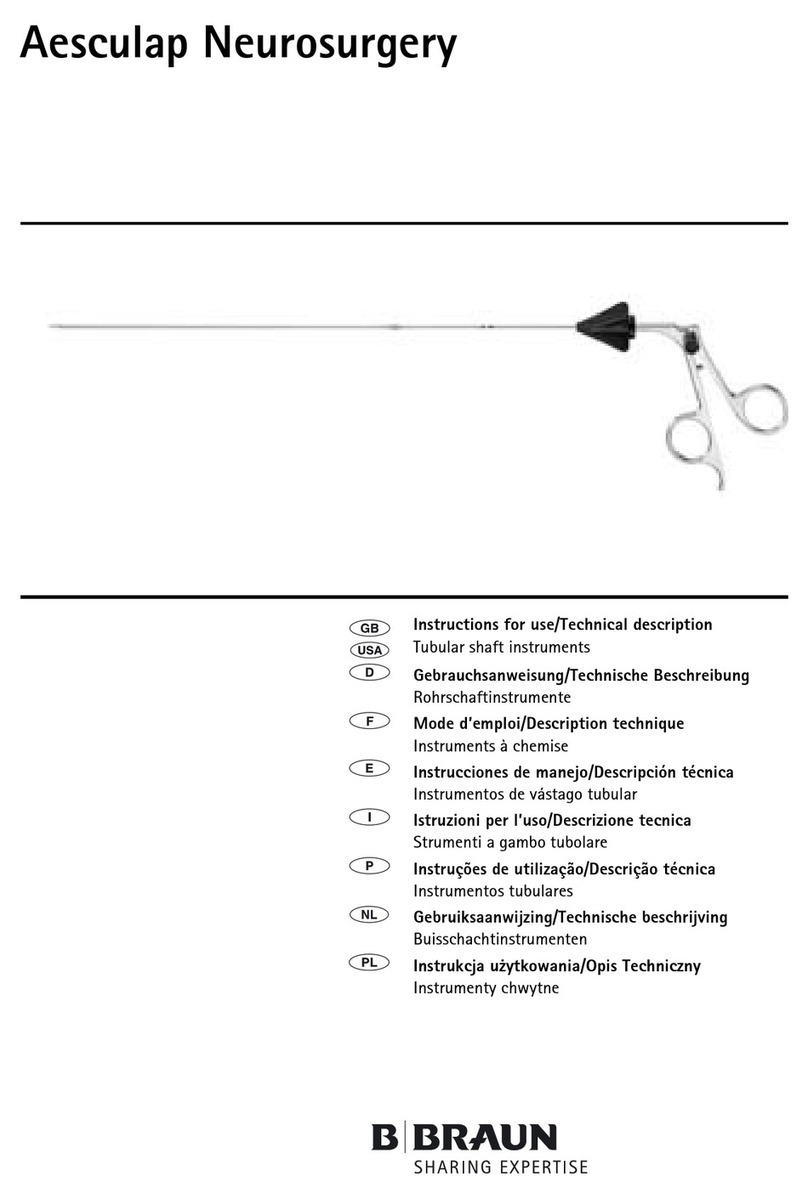
Aesculap®
Système spinal cervical S4® – clé dynamométrique occipitale FW103R
Légende
1Ecrou moleté
2Surface lisse
3Embout hexagonal
4Vis intégrées
Symboles sur le produit et emballage
Domaine d'application
►Pour obtenir le mode d’emploi d’un article ou des informations sur la compatibilité des matériaux, voir aussi
l’extranet d’Aesculap à l’adresse suivante: https://extranet.bbraun.com
Champ d’application
Les clés dynamométriques à déclenchement du système spinal cervical S4servent à serrer les vis de blocage du sys-
tème cervical S4à un couple défini:
■2,8 Nm pour le serrage des vis de blocage dans les têtes de vis/têtes de crochet/plaques occipitales/connecteurs
transversaux et autres connecteurs.
Manipulation sûre et préparation
►Confier le fonctionnement et l’utilisation du produit et des accessoires uniquement à des personnes disposant de
la formation, des connaissances ou de l’expérience requises.
►Lire, observer et conserver le mode d’emploi.
►Utiliser le produit uniquement pour les fins prévues, voir Champ d’application.
►Nettoyer (à la main ou en machine) le produit neuf sortant d’usine après le retrait du conditionnement de trans-
port et avant la première stérilisation.
►Conserver le produit neuf ou non utilisé dans un endroit sec, propre et protégé.
►Avant chaque utilisation, procéder à un examen visuel du produit: absence de pièces lâches, tordues, brisées, fis-
surées, usées et rompues.
►Ne jamais utiliser un produit endommagé ou défectueux. Mettre immédiatement au rebut le produit endommagé.
►Remplacer immédiatement les pièces défectueuses par des pièces de rechange d’origine.
Manipulation
►S’assurer que l’instrument est correctement monté et que l’embout hexagonal est solidement fixé.
►Serrer les vis de blocage en tournant la clé dynamométrique uniformément dans le sens des aiguilles d’une
montre. Ce faisant, veiller à tourner la clé dynamométrique sans saccades.
►L’instrument se déclenche lorsque le couple défini est obtenu.
Démontage
►Dévisser l’écrou moleté 1de l’arrière de la tête de clé, voir Fig. A.
►Retirer l’embout hexagonal 3de la surface intérieure de la tête de clé, voir Fig. B.
►Faire attention de ne pas perdre l’embout hexagonal et l’écrou moleté.
Montage
►Après nettoyage, remonter l’embout hexagonal 3dans la clé dynamométrique en l’insérant dans le logement
prévu à cet effet sur la surface intérieure de la tête de la clé dynamométrique, voir Fig. A.
La surface intérieure de la clé dynamométrique est reconnaissable aux deux vis intégrées 4.
►Engager l’écrou moleté 1à l’arrière de la tête de clé et le serrer fermement, voir Fig. B
L’arrière de la clé est reconnaissable à la surface lisse 2.
Procédé de traitement stérile validé
Consignes générales de sécurité
Remarque
En matière de traitement stérile, respecter les prescriptions légales nationales, les normes et directives nationales et
internationales ainsi que les propres dispositions relatives à l’hygiène.
Remarque
Pour les patients atteints de la maladie de Creutzfeldt-Jakob (CJ), soupçonnés d’être atteints de CJ ou d’éventuelles
variantes, respecter les réglementations nationales en vigueur pour la préparation stérile des produits.
Remarque
Le traitement stérile en machine doit être préféré au nettoyage manuel du fait de résultats de nettoyage meilleurs et
plus fiables.
Remarque
On notera que la réussite du traitement stérile de ce produit médical ne peut être garantie qu’après validation préa-
lable du procédé de traitement stérile. La responsabilité en incombe à l’exploitant/au responsable du traitement sté-
rile.
Pour la validation, les produits chimiques recommandés ont été utilisés.
Remarque
Si aucune stérilisation finale n'a lieu, des produits de décontamination virocides doivent être utilisés.
Remarque
Pour des informations actuelles sur le traitement stérile et la compatibilité avec les matériaux, voir également l’Extra-
net d’Aesculap à l’adresse suivante: https://extranet.bbraun.com
Le procédé validé de stérilisation à la vapeur a été réalisé dans le système de conteneurs stériles Aesculap.
Remarques générales
Les résidus opératoires incrustés ou fixés peuvent faire obstacle au nettoyage ou le rendre inefficace et entraîner
une corrosion. Un intervalle de 6 h entre utilisation et traitement stérile ne devrait par conséquent pas être dépassé,
de même qu’il ne faut pas appliquer de températures de prélavage fixantes >45 °C ni utiliser de produits désinfec-
tants fixants (substance active: aldéhyde, alcool).
Un surdosage du produit de neutralisation ou du détergent de base peut entraîner une agression chimique et/ou le
palissement et l’illisibilité visuelle ou mécanique de l’inscription laser sur l’acier inoxydable.
Sur l’acier inoxydable, les résidus contenant du chlore ou des chlorures (p. ex. les résidus opératoires, médicaments,
solutions salines, eau pour le nettoyage, la décontamination et la stérilisation) entraînent des dégâts dus à la corro-
sion (corrosion perforatrice, sous contrainte) et donc la dégradation des produits. Les résidus sont éliminés par rin-
çage suffisamment abondant à l’eau déminéralisée et séchage consécutif.
Sécher ensuite si nécessaire.
Seuls doivent être utilisés des produits chimiques de traitement contrôlés et validés (p. ex. agrément VAH ou FDA ou
marquage CE) et recommandés par le fabricant des produits chimiques quant à la compatibilité avec les matériaux.
Toutes les prescriptions d’application du fabricant des produits chimiques doivent être strictement respectées. Dans
le cas contraire, les problèmes suivants peuvent survenir:
■Modification d’aspect du matériau, p. ex. palissement ou altérations de couleur du titane ou de l’aluminium. Sur
l’aluminium, des altérations de surface visibles peuvent se produire dès une valeur de pH de >8 dans la solution
utilisée.
■Détériorations de matériau telles que corrosion, fissures, cassures, vieillissement prématuré ou dilatations.
►Pour le nettoyage, ne pas utiliser de brosses métalliques, ni d’autres produits abrasifs pouvant abîmer la surface,
faute de quoi il y a risque de corrosion.
►Pour des informations plus détaillées sur un retraitement hygiéniquement sûr qui ménage les matériaux et
conserve leur valeur aux produits, consulter www.a-k-i.org à la rubrique “Veröffentlichungen Rote Broschüre” -
Le traitement correct des instruments de chirurgie.
Démontage avant l'application du procédé de traitement
►Démonter le produit immédiatement après usage suivant les instructions.
Préparation sur le lieu d’utilisation
►Le cas échéant, rincer les surfaces non visibles, de préférence avec de l'eau déminéralisée, par exemple à l'aide
d'une seringue à usage unique.
►Retirer si possible complètement les résidus opératoires visibles avec un chiffon humide non pelucheux.
►Pour le nettoyage et la décontamination, transporter le produit sec dans un container d’élimination des déchets
fermé dans un délai de 6 h.
Préparation avant le nettoyage
►Démonter le produit avant de le nettoyer, voir Démontage.
Nettoyage/décontamination
Consignes de sécurité spécifiques du produit pour le procédé de traitement
►En cas d’évacuation à l’état humide, utiliser un produit de nettoyage/décontamination adéquat. Pour éviter la
formation de mousse et une dégradation de l’efficacité des produits chimiques de traitement: avant le nettoyage
et la décontamination en machine, rincer abondamment le produit à l’eau courante.
►Procéder au nettoyage aux ultrasons:
– comme traitement mécanique auxiliaire efficace pour compléter le nettoyage/la décontamination manuels.
– comme nettoyage préalable des produits portant des résidus incrustés avant le nettoyage/la décontamination
en machine.
– comme traitement mécanique auxiliaire intégré lors du nettoyage/de la décontamination en machine.
– comme nettoyage consécutif de produits présentant des résidus non éliminés après le nettoyage/la déconta-
mination en machine.
Attention, symbole général de mise en garde
Attention, tenir compte des documents d’accompagnement
Numéro de série du fabricant
AVERTISSEMENT
Risque de blessure/perte de correction en cas de montage impropre de la vis de
blocage!
►Mettre correctement en place la vis de blocage.
►S’assurer que les tiges sont correctement positionnées dans le corps de la tête
de vis polyaxiale.
►Pour le serrage de la vis de blocage, toujours utiliser le stabilisateur prévu à
cet effet pour contrer le couple de serrage.
AVERTISSEMENT
Risque de blessure en cas de montage impropre du connecteur transversal!
►Lors du serrage de la vis de blocage, s’assurer que le connecteur transversal
pose entièrement sur les tiges.
►Serrer la vis de blocage à fond sur le connecteur transversal à l’aide de la clé
dynamométrique.
ATTENTION
Risque d’endommagement de l’implant en cas d’application d’un couple excessif
sur la vis de blocage!
►Serrer lentement la vis de blocage à l’aide de la clé dynamométrique, jusqu’à
ce que cette dernière se déclenche.
ATTENTION
Risque d’endommagement de la clé dynamométrique en cas d’utilisation impropre!
►Pour dévisser une vis de blocage ayant été serrée, toujours utiliser le tournevis
de dépose pour vis de blocage FW064R.
ATTENTION
Risque d’endommagement du stabilisateur en cas d’utilisation impropre!
►Toujours engager le stabilisateur le plus loin possible au-dessus de la tête du
réceptacle de tige. Ce faisant, s’assurer que l’implant s’engage entièrement
dans la fente à l’extrémité de travail.
►S’assurer que la tige dépasse de part et d’autre du stabilisateur.
ATTENTION
Un mauvais montage entraînera un dysfonctionnement de l’instrument!
►S’assurer que l’instrument est correctement monté.
SN
ATTENTION
Risque de détérioration du produit avec un produit de nettoyage/décontamination
inadéquat et/ou des températures trop élevées!
►Utiliser en respectant les instructions du fabricant des produits de nettoyage
et de décontamination
–agréés (p. ex. pour l’aluminium, les plastiques, l’acier inoxydable),
–qui n’attaquent pas les plastifiants (p. ex. en silicone).
►Respecter les indications sur la concentration, la température et le temps
d’action.
►Do not exceed the maximum allowable washing temperature of 55 °C.