USER INFORMATION
User Information shall be provided to the user of the product. NFPA Standard 1983, incorporated into the 2022
edition of NFPA 2500 recommends separating the User Information from the equipment and retaining the
information in a permanent record. The standard also recommends making a copy of the User Information to keep
with the equipment and that the information should be referred to before and after each use.
Additional information regarding life safety equipment can be found in NFPA 1500 and NFPA 1858 and NFPA 1983,
incorporated in the 2022 edition of NFPA 2500.
LIFESPAN / INSPECTION / RETIREMENT
The equipment has a lifespan of 10 years from the date of manufacture shown on the product label. The type of
use, intensity of use, and environment of use are all factors in determining serviceability of the equipment. A single
exceptional event can be cause for retirement after only one use, such as exposure to sharp edges, extreme
temperatures, chemicals, or harsh environments. Any concerns about its safe use is cause for retirement. Remove
retired equipment from service and destroy it to prevent further use.
A device must be retired when:
• It fails to pass inspection.
• It fails to function properly.
• It has illegible product labels or markings.
• It shows signs of damage or excessive wear.
• It has been subjected to shock loads, falls, or abnormal use.
• It has been exposed to harsh chemical reagents.
• It has an unknown usage history.
• You have any doubt as to its condition or reliability.
• When it becomes obsolete due to changes in legislation, standards, technique or incompatibility with other
equipment.
Inspect the equipment according to your department’s policy for inspecting life safety equipment. CMC
recommends a detailed inspection by a competent person at least once every 12 months depending on current
regulations and conditions of use. Record the date, inspector name, and inspection results in the equipment log as
well as any other relevant information to track the usage history.
Before each use, the user should:
• Conrm the equipment is functioning properly.
• Verify the presence and legibility of the product labels and markings.
• Check soft components for cuts, worn or frayed areas, broken bers, soft or hard spots, discoloration, or
melted bers. Check the stitching for pulled threads, abrasion, or breaks.
• Check hard components for excessive wear or indications of damage such as deformation, corrosion, sharp
edges, cracks, or burrs. Minor nicks or sharp spots may be smoothed with emery cloth or similar.
• Check for the presence of dirt or foreign objects that can affect or prevent normal operation such as grit, sand,
rocks, and debris.
During Each Use, the user should:
• Conrm all pieces of equipment in the system are correctly positioned with respect to each other.
• Monitor the condition of the equipment and its connections to other equipment in the system.
• Do not allow anything to interfere with the operation of the equipment or its components.
• Prevent foreign objects from interfering with moving parts.
LIMITATIONS AND PROPER USE
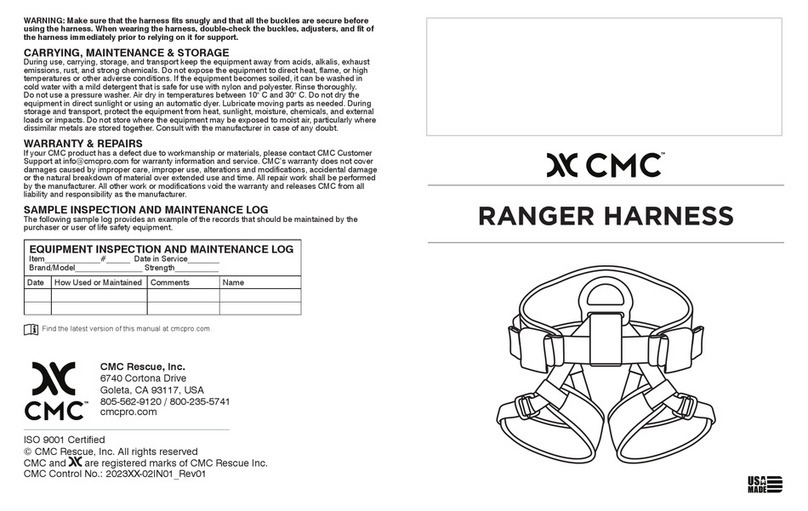
The Drag-N-Lift Harness is designed for victim extrication only and does not provide for any spinal immobilization.
The Drag-N-Lift Harness is compatible with the Oregon Spine Splint II for cases where spinal immobilization
is deemed necessary. Prior to packaging the patient, it is the user’s responsibility to determine whether spinal
immobilization is required and to apply appropriate methods per applicable protocols. See below for additional
product-specic guidance.
PACKAGING THE PATIENT
To package the patient, refer to the steps below and the associated diagram.
1. Disconnect all of the quick-connect buckles and lay the harness with drag sheet as at as possible as near to
the patient as possible. Reverse curl the drag sheet if needed to atten.
2. Slide the patient onto the harness, with the upper gray drag sheet strap positioned just below the armpits.
3. Bring the leg straps up between the patient’s legs, connect the adjustable leg snaphooks into the xed
V-rings (red to red, blue to blue) and pull the webbing until the leg straps are snug. Slide the leg pads as
needed for best comfort.
4. Bring the shoulder straps over each shoulder and connect the adjustable snaps into the xed hip rings (red to
red, blue to blue) and pull the webbing until snug.
5. Connect the sternum strap and pull snug.
6. Connect both of the quick-connect buckles on the gray drag sheet straps and pull to adjust as needed. Make
sure the gray straps run through the black carry handles, not over them. One or both of the patient’s arms may
be left outside of the gray straps as conditions warrant.
7. Prior to any vertical lift, cinch the 1” straps that run from the shoulders to the 1” adjuster buckles on the drag
sheet. This will help maintain a tight drag sheet prole for exit through narrow openings.
WARNING: Make sure that the harness ts snugly and that all the buckles are secure before lifting with the
harness.
USING YOUR EXTRICATION DEVICE
For horizontal drags, use the handles at the center top or bottom of the drag sheet. For horizontal carry, use the
handles at the sides of the drag sheet.
For vertical lifts, connect into both xed V-rings at the shoulders with an appropriate lifting bridle. CMC
recommends the use of a rigid spreader bar no wider than 18 inches.
WARNING: The patient should be removed from horizontal carry or other suspension as quickly as possible.
Precautions should be taken to support and protect the patient’s legs to prevent injury.
Carrying, Maintenance, Storage & Transport
MAX 30˚C
(MAX 86˚F)
MILD MAX 30˚C
(MAX 86˚F)
MAX 30˚C
(MAX 86˚F)
MILD
MAX 30˚C
(MAX 86˚F)
Packaging the Patient
246 5 773