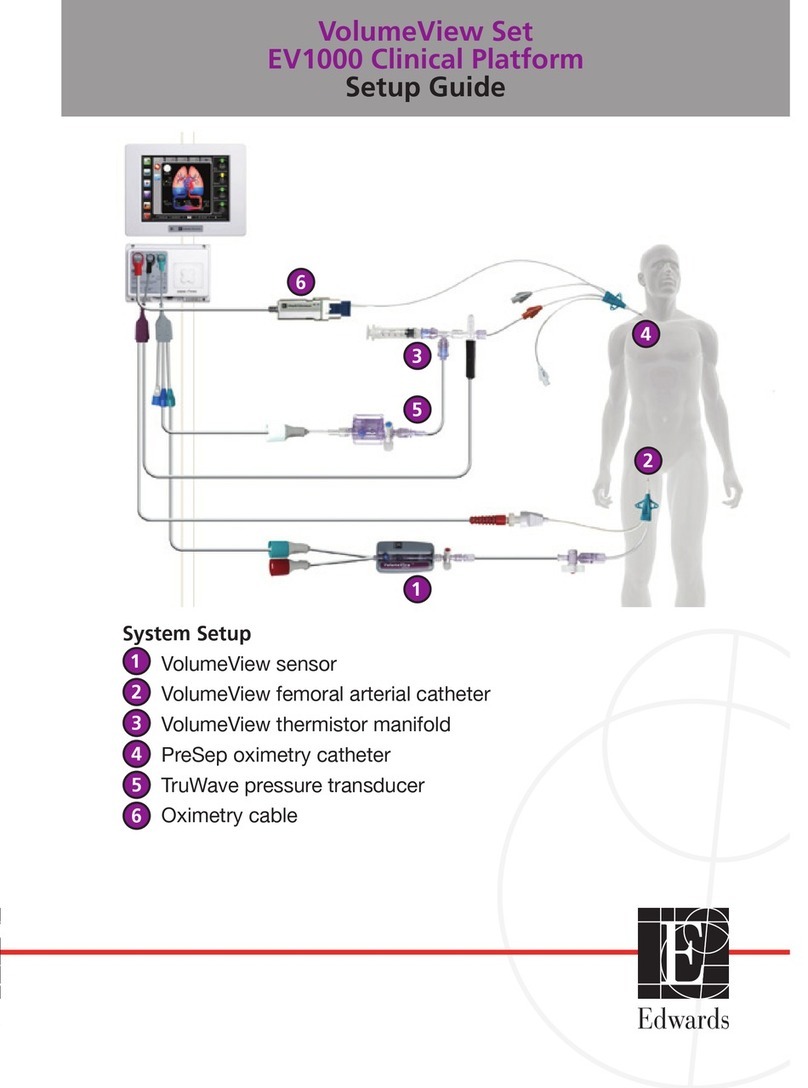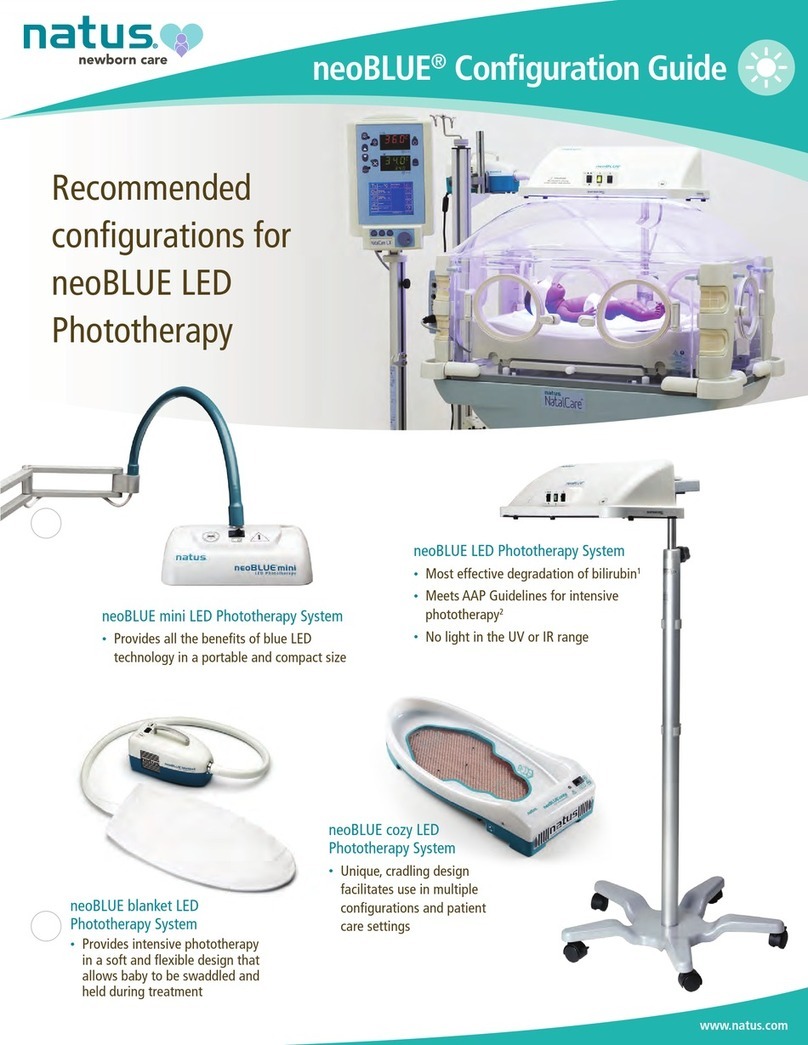
Safety and Symbols 2-5
CAUTION
Inaccurate noninvasive measurements can be caused
by factors such as:
• Improperly zeroed and/or leveled HRS
• Excessive variations in blood pressure. Some con-
ditions that cause BP variations include, but are not
limited to:
* Intra-aortic balloon pumps
• Any clinical situation where the arterial pressure is
deemed inaccurate or not representative of aortic
pressure.
• Poor blood circulation to the fingers
• A bent or flattened ClearSight Finger Cuff
• Excessive patient movement of fingers or hands.
• Artifacts and poor signal quality
• Incorrect placement or position of the ClearSight
Finger Cuff
• Electrocautery or electrosurgical unit interference
(Chapter 7)
CAUTION
Always disconnect the ClearSight Finger Cuff when it is
not wrapped around a finger, to prevent damage by
accidental over-inflation.
(Chapter 7)
CAUTION
The pulsations from intra-aortic balloon support can be
additive to the pulse rate on the instrument pulse rate
display. Verify patient's pulse rate against the ECG
heart rate.
(Chapter 7)
CAUTION
The pulse rate measurement is based on the optical
detection of a peripheral flow pulse and therefore may
not detect certain arrhythmias.The pulse rate should not
be used as a replacement or substitute for ECG based
arrhythmia analysis.
(Chapter 7)
CAUTION
The LIVE DEMO mode can only be initiated by an
Edwards sales representative and is different from
Demo Mode. If a LIVE DEMO banner appears on the
screen, as shown in Figure 11-3, discontinue use the
EV1000 Clinical Platform NI and contact your local
sales representative.
(Chapter 11)
CAUTION
Use Windows Embedded Standard 2009 compatible
USB devices.
(Chapter 11)
CAUTION
Lightly wipe the top, bottom and front surfaces with a
cloth, but the monitor screen and its accessories MUST
NOT have liquid poured or sprayed directly on them. Do
not expose the instrument to excessive moisture.
Excessive moisture can cause the device to perform
inaccurately or fail.
(Appendix E)
CAUTION
Conduct periodic inspections of all cables for defects.
Do not coil cables tightly when storing.
(Appendix E)
CAUTION
If any electrolytic solution, for example NaCl or lactated
Ringer’s solution, is introduced into the cable
connectors while they are connected to the platform, the
excitation voltage can cause electrolytic corrosion and
rapid degradation of the electrical contacts.
(Appendix E)
CAUTION
Do not immerse any cable connectors in fluid or use a
hot air gun to dry cable connectors. Refer to cleaning
instructions.
(Appendix E)
CAUTION
This product contains batteries. If you no longer need to
use this product, protect the environment by bringing it
to your local distributor or designated collection point for
proper disposal.
(Appendix E)
CAUTION
The instrument has been tested and complies with the
limits of IEC 60601-1-2. These limits are designed to
provide reasonable protection against harmful
interference in a typical medical installation. This
equipment generates, uses and can radiate radio
frequency energy and, if not installed and used in
accordance with the instructions, may cause harmful
interference to other devices in the vicinity. However,
there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause
harmful interference to other devices which can be
determined by turning the equipment off and on, the
user is encouraged to try to correct the interference by
one or more of the following measures:
• Reorient or relocate the receiving device.
• Increase the separation between the equipment.
• Consult the manufacturer for help.
(Appendix F)