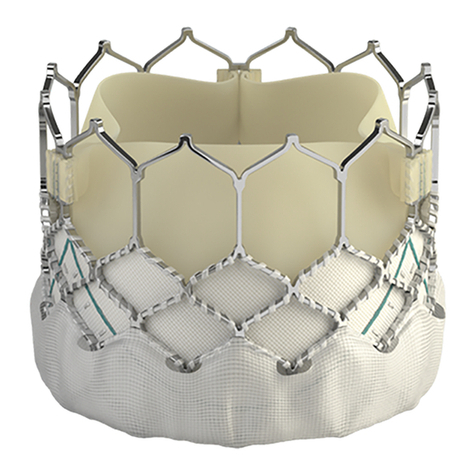
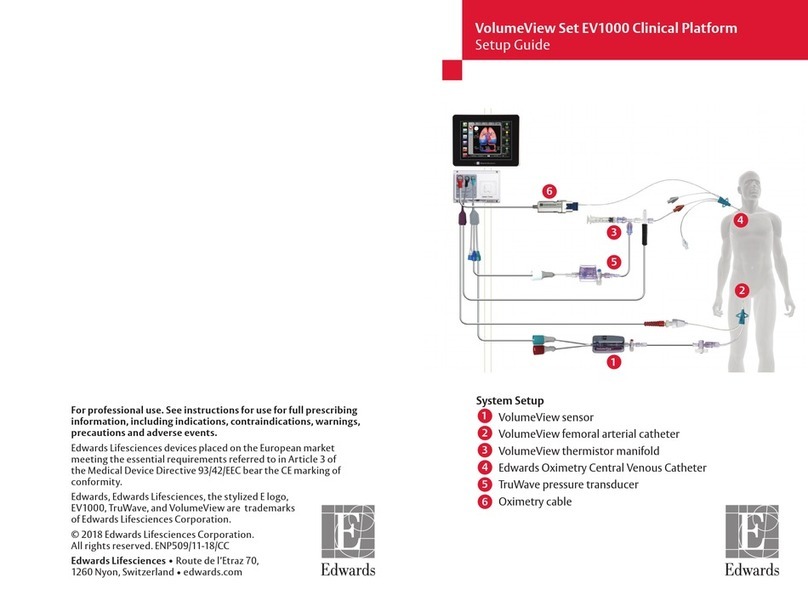
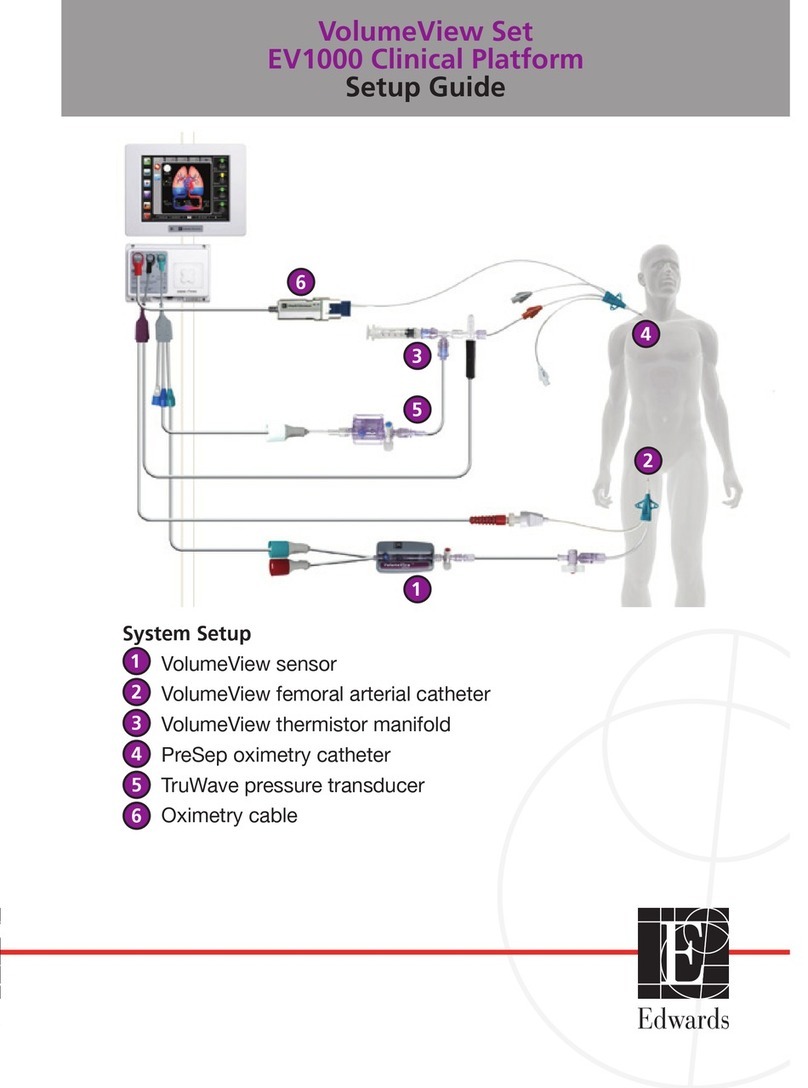
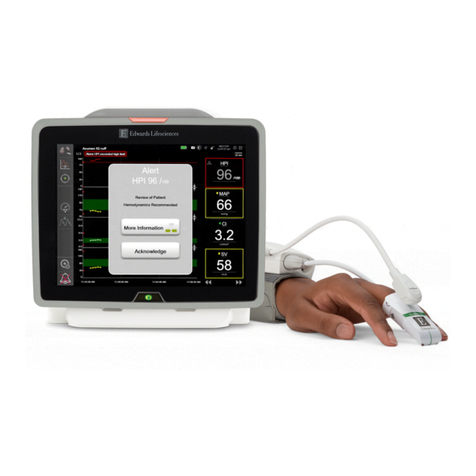
See Vigilance II monitor operator’s manual for detailed instructions.
For professional use. CAUTION: Federal (United States) law restricts this device to sale by or on the
order of a physician. See instructions for use for full prescribing information, including indications,
contraindications, warnings, precautions and adverse events.
Edwards Lifesciences devices placed on the European market meeting the essential requirements referred to
in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity.
Edwards, Edwards Lifesciences, the stylized E logo, Swan-Ganz, Vigilance, and Vigilance II are trademarks of
Edwards Lifesciences Corporation. © 2012 Edwards Lifesciences Corporation. All rights reserved. AR07822
Vigilance II Monitor Quick Reference Guide
Oximetry Setup and Calibration
In-Vitro Calibration
1. Connect the optical module to the SvO2/ScvO2
color-coded connector on the front of the monitor.
Connect catheter to the optical module with the
“Top” side up. Allow 20 minutes for the module
to warm up.
2. Use the navigation knob to highlight the SvO2/
ScvO2large parameter frame. Press knob to
display calibration menu. Highlight In-Vitro
Calibration. Press knob to select.
3. Highlight HGB. Press knob to select. Enter
hospital lab values by turning the knob. Press
knob to confirm setting.
4. Turn knob to highlight Calibrate. Press to select.
Upon successful completion of calibration,
insert catheter in patient.
5. Highlight Start. Press knob to select.
In-Vivo Calibration
1. Use the navigation knob to highlight the SvO2/
ScvO2large parameter frame. Press knob to
display calibration menu.
2. Highlight In-Vivo Calibration. Press knob to select.
3. After 25 seconds Draw is highlighted. Press knob
just prior to drawing waste and lab samples.
4. Use knob to enter oximetry and HGB or Hct
values.
5. Highlight Calibrate. Press knob to select.
To Transport
1. The Swan-Ganz catheter should remain
connected to the optical module during transport.
Unplug the optical module cable from the
Vigilance II monitor. Transport the patient.
2. If reconnecting to a different Vigilance II monitor,
press the patient data button, select New Patient,
select Yes, and Yes again.
3. Reconnect the optical module cable to the
Vigilance II monitor.
4. Highlight and select oximetry large parameter
frame. Select Recall OM Data. The calibration
data stored in the optical module will be
displayed.
5. If stored data is less than 24 hours old a Yes or No
option will be displayed. Select Yes to begin
oximetry monitoring. If No is selected, In-Vivo
calibration will be necessary.
Continuous Cardiac Output
1. Connect the patient CCO cable to the color-
coded connector on the front panel.
2. If CEDV is required, connect the ECG interface
cable from the bedside monitor to the ECG
connector on the Vigilance II monitor rear panel.
3. Connect an Edwards advanced technology
Swan-Ganz catheter to the patient cable per
catheter instructions.
4. Press CO Start/Stop button to begin continuous
cardiac output monitoring. If CEDV monitoring
is required:
•Highlightoneofthelargeparameterframes.
Press knob to display menu.
•HighlightandselectParameter, then EDV.
Highlight Return. Push knob to select.
Activating STAT Boxes
1. Highlight the Full/Split Screen icon.
Press knob to select.
2. Highlight the lower frame of the split screen.
Press knob to select.
3. Highlight STAT Boxes.
Press knob to select.
Signal Quality
Signal Quality May be Compromised by:
•Pulsatility
•Signalintensity(e.g.,kinkingofcatheter,
blood clot, hemodilution)
•Intermittentwallcontactbythecatheter
Signal Quality May be Improved by:
•Trytoaspiratedistallumen;ifabletoaspirate,
flush lumen with extreme caution
•Checkcatheterforkinkingandrecalibrate;
replace catheter if required and recalibrate
•RepositioncatheterandifSQI>2,recalibrate
monitor by performing in-vivo calibration
•Attempttodistanceelectrocauteryequipment
and cables from the Vigilance monitor
•PlugthepowercordintoseparateACcircuits
if possible
•Updateenteredhemoglobinandhematocrit
values when there is a physiologic change of
6% or greater in hematocrits or of 1.8 g/dl
(1.1mmol/L)orgreaterinhemoglobin
Edwards Lifesciences
Irvine, USA INyon, Switzerland ITokyo, Japan ISingapore, Singapore ISão Paulo, Brazil
edwards.com