Edwards SAPIEN XT User manual

1
Edwards SAPIEN XT
Transcatheter Heart Valve with the Ascendra+ Delivery System
Instructions for Use
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
Implantation of the transcatheter heart valve should be performed only by physicians who
have received Edwards Lifesciences training. The implanting physician should be experienced
in balloon aortic valvuloplasty.
Please verify that you have the latest version of the instructions for use prior to using the
device by visiting http://THVIFU.edwards.com or by calling 1.800.822.9837. In order to access
the instructions for use, an IFU Code will be required.
STERILE: The valve is supplied sterilized with glutaraldehyde solution. The delivery system is
supplied sterilized with ethylene oxide gas.
_________________________________________________________________________________________
Edwards, Edwards Lifesciences, the stylized E logo, Ascendra, Ascendra+, Carpentier-Edwards,
Edwards SAPIEN, Edwards SAPIEN XT, PARTNER and PARTNER II, SAPIEN, SAPIEN XT, TFX, and
ThermaFix are trademarks of Edwards Lifesciences Corporation. All other trademarks are the property of their
respective owners.

2
1.0 Device Description
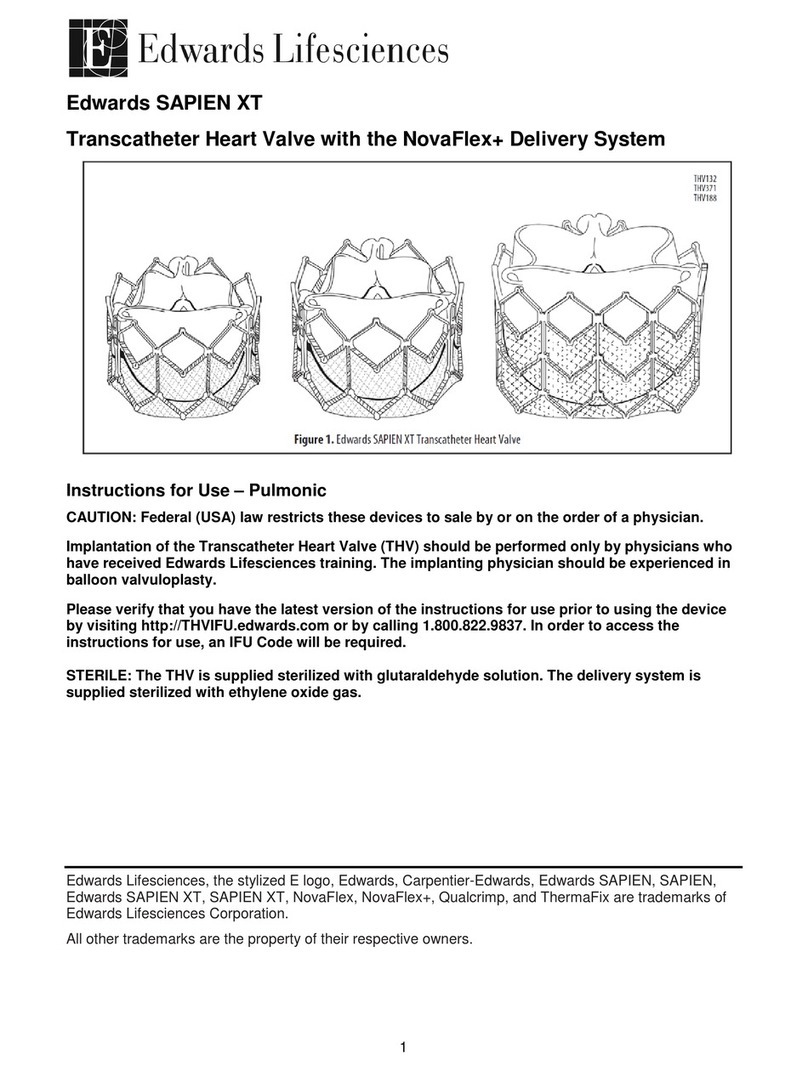
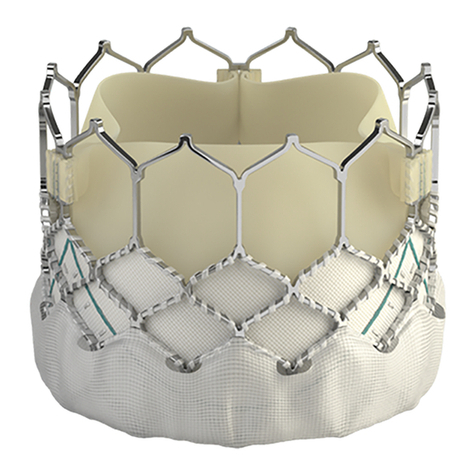
•Edwards SAPIEN XT Transcatheter Heart Valve – Model 9300TFX (Figure 1)
The Edwards SAPIEN XT transcatheter heart valve is comprised of a balloon-expandable,
radiopaque, cobalt-chromium frame, trileaflet bovine pericardial tissue valve, and a polyethylene
terephthalate (PET) fabric skirt. The leaflets are treated according to the Carpentier-Edwards
ThermaFix process.
Table 1
Valve Size Height
23 mm 14.3 mm
26 mm 17.2 mm
29 mm 19.1 mm
Table 2
Native Valve Annulus
Size
(TEE)
Native Valve Annulus Size
(CT) Valve Size
Area Area Derived
Diameter
18-22 mm 314 – 415 mm
2
20-23 mm 23 mm
21-25 mm 415 – 530 mm
2
23-26 mm 26 mm
24-27 mm 530 – 660 mm2 26-29 mm 29 mm
Valve size recommendations are based on native valve annulus size, as measured by
transesophageal echocardiography (TEE) or computed tomography (CT). Patient anatomical
factors and multiple imaging modalities should be considered during valve size selection.
Note: Risks associated with undersizing and oversizing should be considered.
For transcatheter valve-in-surgical valve procedures, size recommendations for surgical
bioprostheses with internal orifice diameters are shown in Table 3.
Table 3
Bioprosthesis Internal Orifice
Diameter
SAPIEN XT Valve Size
18-21 mm
23 mm
21-23.5 mm
26 mm
23.5-27 mm
29 mm
NOTE: The internal orifice diameter of the surgical bioprosthesis must be determined so
that the appropriate valve size can be implanted. The bioprosthesis internal diameter of
the primary implanted device is best determined by using computed tomography,
magnetic resonance imaging, and/or transesophageal echocardiography to perform the
necessary measurements. The internal orifice diameter is a directly measured or area
derived diameter measurement of the internal opening of the failed surgical valve.
NOTE: Exact volume required to deploy the valve may vary depending on the
bioprosthesis internal orifice diameter. Do not exceed the rated burst pressure.
See Table 4 for inflation parameters.

3
•Ascendra+ Delivery System (Figures 2a, 2b, 2c)
The Ascendra+ delivery system (useable length 55 cm) is used for delivery of the
Edwards SAPIEN XT transcatheter heart valve. The delivery system has radiopaque markers
for visualization under fluoroscopy and a balloon for deployment of the valve. A balloon inflation
hub, a guidewire hub, and a pusher retraction feature are housed in the handle assembly. The
handle is labeled “BALLOON” at the balloon inflation hub and “WIRE 0.035"” at the guidewire
hub. The system also comes with a loader that is used to cover the valve during delivery. An
extension tube is supplied for use with the delivery system during inflation.
Table 4
Model Nominal
Balloon
Diameter
Nominal
Inflation
Volume
Rated Burst
Pressure
(RBP)
9355AS23 23 mm 16 mL 7 atm
9355AS26 26 mm 20 mL 7 atm
9355AS29 29 mm 30 mL 7 atm
2.0 Indications
The Edwards SAPIEN XT transcatheter heart valve, model 9300TFX, and accessories are indicated
for relief of aortic stenosis in patients with symptomatic heart disease due to severe native calcific
aortic stenosis who are judged by a Heart Team, including a cardiac surgeon, to be at intermediate or
greater risk for open surgical therapy (i.e., predicted risk of surgical mortality ≥ 3% at 30 days, based
on the Society of Thoracic Surgeons (STS) risk score and other clinical co-morbidities unmeasured by
the STS risk calculator).

4
The Edwards SAPIEN XT transcatheter heart valve and accessories are also indicated for patients
with symptomatic heart disease due to failure (stenosed, insufficient, or combined) of a surgical
bioprosthetic aortic valve who are judged by a heart team, including a cardiac surgeon, to be at high or
greater risk for open surgical therapy (i.e., STS operative risk score ≥8% or at a ≥15% risk of mortality
at 30 days).
3.0 Contraindications
The valve and delivery system are contraindicated in patients who cannot tolerate an
anticoagulation/antiplatelet regimen or who have active bacterial endocarditis or other active
infections.
4.0 Warnings
•Observation of the pacing lead throughout the procedure is essential to avoid the potential risk
of pacing lead perforation.
•There may be an increased risk of stroke in transcatheter aortic valve replacement
procedures, as compared to balloon aortic valvuloplasty or other standard treatments in high
or greater risk patients.
•The devices are designed, intended, and distributed for single use only. Do not resterilize or
reuse the devices. There are no data to support the sterility, nonpyrogenicity, and
functionality of the devices after reprocessing.
•Care should be exercised when sizing the native annulus or surgical valve; implanting a valve
that is too small may lead to paravalvular leak, migration or embolization, whereas implanting
a valve that is too large may lead to residual gradient (patient-prosthesis mismatch) or
annular rupture.
•Accelerated deterioration of the valve may occur in patients with an altered calcium
metabolism.
•Prior to delivery, the valve must remain hydrated at all times and cannot be exposed to
solutions other than its shipping storage solution and sterile physiologic rinsing solution. Valve
leaflets mishandled or damaged during any part of the procedure will require replacement of
the valve.
•Caution should be exercised in implanting a valve in patients with clinically significant coronary
artery disease.
•Patients with pre-existing mitral valve devices should be carefully assessed prior to
implantation of the valve to ensure proper valve positioning and deployment.
•Patients presenting with combination AV low flow, low gradient should undergo additional
evaluation to establish the degree of aortic stenosis.
•Do not use the valve if the tamper evident seal is broken, the storage solution does not
completely cover the valve, the temperature indicator has been activated, the valve is
damaged, or the expiration date has elapsed.
•Do not mishandle the Ascendra+ delivery system or use it if the packaging or any components
are not sterile, have been opened or are damaged (e.g. kinked or stretched), or the expiration
date has elapsed.
•Care should be exercised in patients with hypersensitivities to cobalt, nickel, chromium,
molybdenum, titanium, manganese, silicon, and/or polymeric materials.
•The procedure should be conducted under fluoroscopic guidance. Some fluoroscopically
guided procedures are associated with a risk of radiation injury to the skin. These injuries may
be painful, disfiguring, and long-lasting.

5
•Valve recipients should be maintained on anticoagulant/antiplatelet therapy, except when
contraindicated, as determined by their physician. This device has not been tested for use
without anticoagulation.
•Do not add or apply antibiotics to the storage solution, rinse solutions, or to the valve.
5.0 Precautions
•Long-term durability has not been established for the valve. Regular medical follow-up is
advised to evaluate valve performance.
•Glutaraldehyde may cause irritation of the skin, eyes, nose and throat. Avoid prolonged or
repeated exposure to, or breathing of, the solution. Use only with adequate ventilation. If skin
contact occurs, immediately flush the affected area with water; in the event of contact with
eyes, seek immediate medical attention. For more information about glutaraldehyde exposure,
refer to the Material Safety Data Sheet available from Edwards Lifesciences.
•To maintain proper valve leaflet coaptation, do not overinflate the deployment balloon.
•Appropriate antibiotic prophylaxis is recommended post-procedure in patients at risk for
prosthetic valve infection and endocarditis.
•Safety, effectiveness, and durability have not been established for transcatheter valve in
transcatheter valve procedures.
•Safety and effectiveness have not been established for patients with the following
characteristics/comorbidities:
oNon-calcified aortic annulus
oSevere ventricular dysfunction with ejection fraction < 20%
oCongenital unicuspid or congenital bicuspid aortic valve
oMixed aortic valve disease (aortic stenosis and aortic regurgitation with predominant
aortic regurgitation > 3+)
oPre-existing prosthetic ring in any position
oSevere mitral annular calcification (MAC), severe (> 3+) mitral insufficiency, or Gorlin
syndrome
oBlood dyscrasias defined as: leukopenia (WBC < 3000 cells/mL), acute anemia
(Hb < 9 g/dL), thrombocytopenia (platelet count < 50,000 cells/mL), or history of bleeding
diathesis or coagulopathy
oHypertrophic cardiomyopathy with or without obstruction (HOCM)
oEchocardiographic evidence of intracardiac mass, thrombus, or vegetation
oA known hypersensitivity or contraindication to aspirin, heparin, ticlopidine (Ticlid™), or
clopidogrel (Plavix™), or sensitivity to contrast media, which cannot be adequately
premedicated
oExcessive calcification of vessel at access site
oBulky calcified aortic valve leaflets in close proximity to coronary ostia
oA concomitant paravalvular leak where the surgical bioprosthesis is not securely fixed in
the native annulus or is not structurally intact (e.g. wireform frame fracture)
oA partially detached leaflet of the surgical bioprosthesis that in the aortic position may
obstruct a coronary ostium

6
•The safety and effectiveness have not been established for implanting the transcatheter valve
inside a stented bioprosthetic valve < 21 mm (labeled size) or an unstented bioprosthetic
aortic valve.
•Residual mean gradient may be higher in a "TAV-in-SAV" configuration than that observed
following implantation of the valve inside a native aortic annulus using the same size device.
Patients with elevated mean gradient post procedure should be carefully followed. It is
important that the manufacturer, model and size of the preexisting surgical bioprosthetic aortic
valve be determined, so that the appropriate valve can be implanted and a prosthesis-patient
mismatch be avoided. Additionally, pre-procedure imaging modalities must be employed to
make as accurate a determination of the internal orifice as possible.
6.0 Potential Adverse Events
Potential risks associated with the overall procedure including potential access complications
associated with standard cardiac catheterization, balloon valvuloplasty, the potential risks of
conscious sedation and/or general anesthesia, and the use of angiography:
•Death
•Stroke/transient ischemic attack, clusters or neurological deficit
•Paralysis
•Permanent disability
•Respiratory insufficiency or respiratory failure
•Hemorrhage requiring transfusion or intervention
•Cardiovascular injury including perforation or dissection of vessels, ventricle, myocardium or
valvular structures that may require intervention
•Pericardial effusion or cardiac tamponade
•Embolization including air, calcific valve material or thrombus
•Infection including septicemia and endocarditis
•Heart failure
•Myocardial infarction
•Renal insufficiency or renal failure
•Conduction system defect which may require a permanent pacemaker
•Arrhythmia
•Retroperitoneal bleed
•AV fistula or pseudoaneurysm
•Reoperation
•Ischemia or nerve injury
•Restenosis
•Pulmonary edema
•Pleural effusion
•Bleeding
•Anemia
•Abnormal lab values (including electrolyte imbalance)

7
•Hypertension or hypotension
•Allergic reaction to anesthesia, contrast media, or device materials
•Hematoma
•Syncope
•Pain or changes at the access site
•Exercise intolerance or weakness
•Inflammation
•Angina
•Heart murmur
•Fever
Additional potential risks associated with the use of the valve, delivery system, and/or accessories
include:
•Cardiac arrest
•Cardiogenic shock
•Emergency cardiac surgery
•Cardiac failure or low cardiac output
•Coronary flow obstruction/transvalvular flow disturbance
•Device thrombosis requiring intervention
•Valve thrombosis
•Device embolization
•Device migration or malposition requiring intervention
•Valve deployment in unintended location
•Valve stenosis
•Structural valve deterioration (wear, fracture, calcification, leaflet tear/tearing from the stent
posts, leaflet retraction, suture line disruption of components of a prosthetic valve, thickening,
stenosis)
•Device degeneration
•Paravalvular or transvalvular leak
•Valve regurgitation
•Hemolysis
•Device explants
•Nonstructural dysfunction
•Mechanical failure of delivery system, and/or accessories
•Non-emergent reoperation

8
7.0 Directions for Use
7.1 Required Equipment
Table 5
Product Name 23 mm System
(9355ASP23A) 26 mm System
(9355ASP26A) 29 mm System
(9355ASP29A)
Model
Edwards SAPIEN XT
Transcatheter Heart Valve 9300TFX (23 mm) 9300TFX (26 mm) 9300TFX (29 mm)
Ascendra+ Delivery System* 9355AS23 9355AS26 9355AS29
Ascendra+ Introducer Sheath
Set 9350IS23 9350IS26 9350IS29
Ascendra Balloon Aortic
Valvuloplasty Catheter 9100BAVC
Inflation devices provided by Edwards Lifesciences
Edwards Crimper 9350CR
* Includes the Crimp Stopper
Additional Equipment:
•20 cc syringe or larger (x2)
•50 cc syringe or larger
•Standard cardiac catheterization lab equipment
•Fluoroscopy (fixed, mobile or semi-mobile fluoroscopy systems appropriate for use in
percutaneous coronary interventions)
•Transesophageal or transthoracic echocardiography capabilities
•Exchange length 0.035 inch (0.89 mm) extra-stiff guidewire
•Temporary pacemaker (PM) and pacing lead
•Sterile rinsing basins, physiological saline, heparinized saline, 15% diluted radiopaque contrast
medium
•Sterile table for valve and device preparation
7.2 Valve Handling and Preparation
Follow sterile technique during device preparation and implantation.

9
7.2.1 Valve Rinsing Procedure
Before opening the valve jar, carefully examine for evidence of damage (e.g., a cracked jar or lid,
leakage, or broken or missing seals).
CAUTION: Valves from containers found to be damaged, leaking, without adequate sterilant,
or missing intact seals must not be used for implantation.
Step Procedure
1 Set up two (2) sterile bowls with at least 500 mL of sterile physiological saline to thoroughly rinse the
glutaraldehyde sterilant from the valve.
2 Carefully remove the valve/holder assembly from the jar without touching the tissue. Verify the valve
serial identification number with the number on the jar lid and record in the patient information
documents. Inspect the valve for any signs of damage to the frame or tissue.
3
Rinse the valve as follows: Place the valve in the first bowl of sterile, physiological saline. Be sure the
saline solution completely covers the valve and holder. With the valve and holder submerged, slowly
agitate (to gently swirl the valve and holder) back and forth for a minimum of 1 minute. Transfer the
valve and holder to the second rinsing bowl of physiological saline and gently agitate for at least one
more minute. Ensure the rinse solution in the first bowl is not used. The valve should be left in the final
rinse solution until needed to prevent the tissue from drying.
CAUTION: Do not allow the valve
to come into contact with the bottom or sides of the rinse bowl
during agitation or swirling in the rinse solution. Direct contact between the identification tag
and valve is also to be avoided during the rinse procedure. No other objects should be placed in
the rinse bowls. The valve should be kept hydrated to prevent the tissue from drying.
7.2.2 Prepare the Components
Step Procedure
1 Visually inspect all components for damage.
2 Refer to Ascendra+ Introducer Sheath Set and Crimper instructions for use on device preparation and
handling.
3 Ensure the delivery system pusher is in the distal locked position using the slider cap. If the stopcock is
not attached to the delivery system, attach stopcock to the flush port. Flush delivery system at the flush
port with heparinized saline and close stopcock to delivery system.
4 Carefully remove distal balloon cover.
5
Flush loader through the distal end with heparinized saline and insert the delivery system (with proximal
balloon cover on) into loader until loader is completely proximal.
6 Fully retract slider cap and rotate into proximal slot.
7 Slide the proximal balloon cover onto the balloon shaft and carefully peel off the proximal balloon cover
from the delivery system.
8 Flush and attach balloon extension tube to the balloon inflation hub.
9 Prepare a 50 cc or larger luer-lock syringe with diluted contrast solution (15:85 contrast to heparinized
saline) and attach to the extension tubing.
10 Completely fill the inflation device provided by Edwards with diluted contrast and attach to the
extension tubing stopcock. Ensure there are no air bubbles in the balloon. If an air bubble is detected,
eliminate it while deflating the balloon. Close the stopcock to the delivery system.

10
Step Procedure
11
Remove excess contrast medium from the inflation device provided by Edwards into the syringe to
achieve the appropriate volume required to deploy the valve per the following. Then lock the inflation
device:
Delivery System Valve Inflation
Volume
Model 9355AS23 23 mm 16 mL
Model 9355AS26 26 mm 20 mL
Model 9355AS29 29 mm 30 mL
Note: Correct balloon sizing is critical to successful valve deployment and valve function.
12 Close the stopcock to the 50 cc or larger syringe and remove the syringe.
CAUTION: Maintain the inflation device provided by Edwards in the locked position until valve
deployment.
7.2.3 Mount and Crimp the Valve onto the Delivery System
Step Procedure
1 Rotate the crimper until the aperture is fully opened.
2 Remove the valve from the holder and remove ID tag using sterile scissors.
3 Place valve into crimper aperture and partially crimp so that it fits loosely over the prepared balloon.
4
Remove the valve from the crimper and place it on the delivery system with the inflow (fabric cuff end)
of the valve proximally towards the pusher if accessing antegrade. If accessing retrograde, place the
valve on the delivery system with the inflow (fabric cuff end) of the valve towards the distal end away
from the pusher. Ensure that the valve is aligned between the radiopaque markers.
5
Place the valve/balloon assembly in crimper aperture and gradually crimp. Periodically open crimper to
verify correct placement of valve during crimping. Completely crimp until the handle contacts the crimp
stopper.
CAUTION: The implanting physician must verify correct mounting/orientation of the valve prior
to its implantation.
6 Advance the slider cap distally to allow the tip of the pusher to align with the proximal end of the
crimped valve.
7 Advance the loader onto crimped valve until it reaches the balloon shoulder and the valve is fully
covered.
8
While holding the loader in place, fully retract the slider cap and rotate into locked position. Flush
through the flush port to fill the loader and hydrate the valve. Once the valve is hydrated, advance the
slider cap and rotate into distal locked position. Be sure to maintain position of the crimped valve
between the radiopaque markers during hydration. Close the flush port stopcock to the delivery system.
Note: To facilitate flushing, keep the delivery system straight.
CAUTION: To prevent possible leaflet damage, the valve should not remain in the loader over 30
minutes.
9 Ensure the slider cap is locked in the distal position and that the valve is still centered between
radiopaque markers and fully inside the loader.
Note: Keep valve hydrated until ready for implantation.

11
Step Procedure
10 Remove the stylet and flush the guidewire lumen of the delivery system.
CAUTION: The implanting physician must verify correct orientation of the valve prior to its
implantation.
7.3 Valvuloplasty and Valve Delivery
Valvuloplasty and valve delivery should be performed under general anesthesia with hemodynamic
monitoring in a catheterization lab/hybrid operating room with fluoroscopic and echocardiographic
imaging capabilities.
The following table shows the minimum required distances from the native valve annulus to the distal
tip of the Ascendra+ sheath to allow the Ascendra+ delivery system balloon to inflate properly during
valve deployment. These distances should be considered during the transaortic approach when
selecting the access site on the ascending aorta and determining the insertion depth of the
Ascendra+ sheath into the aorta.
Delivery System Valve
Minimum Required
Distance From
Annulus to Sheath Tip
Model 9355AS23
23 mm
5.0 cm
Model 9355AS26
26 mm
5.5 cm
Model 9355AS29
29 mm
6.0 cm
Administer heparin to maintain the ACT at ≥ 250 sec.
CAUTION: Contrast media use should be monitored to reduce the risk of renal injury.
7.3.1 Baseline Parameters
Step Procedure
1 Perform an angiogram with fluoroscopic view perpendicular to the valve.
2 Evaluate the height between the inferior aspect of the annulus and the inferior aspects of the lowest
coronary ostium for subsequent prosthetic aortic valve implantation.
3 Introduce a pacemaker (PM) lead until its distal end is positioned in the right ventricle.
4 Set the stimulation parameters, and test pacing.
7.3.2 Valvuloplasty
Refer to Ascendra Balloon Aortic Valvuloplasty Catheter Instructions for Use (IFU) for information on
device preparation and handling for a stenotic aortic valve.
Note: Rapid ventricular pacing should be performed when using the Ascendra balloon aortic
valvuloplasty catheter for valvuloplasty prior to transcatheter valve implantation.
After placement of the balloon at the intended site, begin rapid ventricular pacing. Once the blood
pressure has decreased to 50 mmHg or below, balloon inflation can commence.
CAUTION: Valve implantation should not be carried out if the balloon cannot be fully inflated
during valvuloplasty.

12
7.3.3 Valve Delivery
Step Procedure
1 Insert the introducer sheath. Refer to the Ascendra+ Introducer Sheath Set IFU for additional
information on device preparation and handling.
2 Advance delivery system over guidewire. Engage loader into introducer sheath housing while
maintaining a firm grip. Tap lightly on the introducer sheath housing to release air to the proximal end of
the loader. Lightly depress button valves on loader to aspirate the loader.
3 Cross the native aortic valve or bioprosthesis and position the transcatheter valve within the valve.
4
Retract pusher by rotating slider cap out of distal locked position and moving it proximally to ensure that
the tip of the pusher is retracted completely on to the balloon shaft.
CAUTION: The pusher must be pulled back completely on the balloon shaft for proper balloon
inflation and valve deployment.
5 Verify the correct position of the valve with respect to the valve.
6
Begin valve deployment:
• Unlock the inflation device.
• Begin rapid pacing; once arterial blood pressure has decreased to 50 mmHg or below,
balloon inflation can commence.
• Deploy the valve by inflating the balloon with the entire volume in the Inflation device provided by
Edwards Lifesciences, hold for 3 seconds and confirm that the barrel of the inflation device is empty to
ensure complete inflation of the balloon. When the balloon catheter has been completely deflated, turn
off the pacemaker.
• Retract the delivery system into the introducer sheath.
7 Disengage loader from sheath and remove delivery system and loader.
8 Remove sheath when the ACT level is appropriate (e.g. reaches < 150 sec). Close access site.
8.0 How Supplied
STERILE: The valve is supplied sterilized with glutaraldehyde solution. The delivery system is
supplied sterilized with ethylene oxide gas.
8.1 Storage
The valve must be stored at 10 °C to 25 °C (50 °F to 77 °F). Each jar is shipped in an enclosure
containing a temperature indicator to detect exposure of the valve to extreme temperature.
The delivery system should be stored in a cool, dry place.
9.0 MR Safety
MR Conditional
Non-clinical testing has demonstrated that the Edwards SAPIEN XT transcatheter heart valve is MR
Conditional. A patient with this device, when implanted in the native valve or a failed surgical
bioprosthesis, can be scanned safely, immediately after placement of this device under the following
conditions:
•Static magnetic field of 1.5 tesla or 3 tesla
•Maximum spatial gradient field of 2500 gauss/cm (25 T/m) or less
•Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 2 W/kg
(Normal Operating Mode)
Under the scan conditions defined above, the SAPIEN XT transcatheter heart valve is expected to
produce a maximum temperature rise of 2.6 °C after 15 minutes of continuous scanning.

13
In non-clinical testing, the image artifact caused by the device extends as far as 14.5 mm from the
implant for spin echo images and 30 mm for gradient echo images when scanned in a 3.0 T MRI
system. The artifact obscures the device lumen in gradient echo images.
The implant has not been evaluated in MR systems other than 1.5 or 3.0 T.
For valve-in-surgical valve implantation or in the presence of other implants, please refer to MRI
safety information for the surgical valve or other devices prior to MR imaging.
10.0 Patient Information
Patient education brochures are provided to each site and should be given to the patient to inform
them of the risks and benefits of the procedure and alternatives in adequate time before the
procedure to be read and discussed with their physician. A copy of this brochure may also be
obtained from Edwards Lifesciences by calling 1.800.822.9837. A patient implant card request form is
provided with each transcatheter heart valve. After implantation, all requested information should be
completed on this form. The serial number may be found on the package and on the identification tag
attached to the transcatheter heart valve. The original form should be returned to the
Edwards Lifesciences address indicated on the form and upon receipt, Edwards Lifesciences will
provide an identification card to the patient.
11.0 Recovered valve and Device Disposal
The explanted valve should be placed into a suitable histological fixative such as 10% formalin or 2%
glutaraldehyde and returned to the company. Refrigeration is not necessary under these
circumstances. Contact Edwards Lifesciences to request an Explant Kit.
Used delivery system may be disposed of in the same manner that hospital waste and biohazardous
materials are handled. There are no special risks related to the disposal of these devices.
12.0 Clinical Studies
The PARTNER II Cohort B Registries
Cohort B of The Placement of Aortic Transcatheter Valves Trial II (PARTNER II) included registries
for the transapical and transaortic delivery of the SAPIEN XT valve. These registries include the
following:
•NR1: Inoperable Transapical (TA) Registry – transapical delivery of the 23 mm or
26 mm SAPIEN XT valve.
•NR3: Registry for Transcatheter Heart Valve in Aortic Surgical Valve Implantation (THV-
SV). Patients with failing aortic bioprosthetic surgical valve with a surgical mortality or major
morbidity ≥ 50% and meeting the sizing requirements for 23 mm or 26 mm SAPIEN XT valve.
•NR4: Inoperable Transaortic (TAo) Registry – transaortic delivery of the 23 mm or 26 mm
SAPIEN XT valve.
•NR6: Inoperable Transapical Registry for the delivery of 29 mm SAPIEN XT valve in patients
that did not have eligible transfemoral access.
Following completion of enrollment in the nested registries, the FDA approved continued access
enrollment in the nested registries (CANRs).
SOURCE Registry XT:
SOURCE Registry XT is an international multi-center prospective, consecutively enrolled,
observational registry. Consecutive patient data have been collected at discharge, 30 days, and 12
months post-implant, and will be collected annually thereafter up to 5 years post-implant.
Results of PARTNER II Cohort B Registries (NR1, NR4 and NR6)
A total of 265 patients were treated in PARTNER II Cohort B Nested Registries 1, 4, and 6. The
primary safety and effectiveness endpoint was freedom from all-cause mortality at 1 year. The KM
estimate at 30 days involving freedom from all-cause mortality was 92.0 ± 1.7%.

14
There were 1.9% major strokes, no incidence of endocarditis, 1.5% myocardial infarction, 5.7% major
vascular complications, 11.3% disabling bleeding events, 3.0% cardiac intervention, and 4.5% new
pacemaker at 30 days.
NYHA went from 3.2 ± 0.61 at baseline to 1.9 ± 0.88 at 30 days. The mean change was -1.3 ± 1.10.
Device success was observed in 69.6% of patients (165/237). The mean hospitalization stay was
11.1 ± 8.96 days which included 4.5 ± 7.12 days in the ICU. The mean EOA was 0.7 ± 0.19 cm2at
baseline and 1.6 ± 0.43 cm2at 30 days, and the average mean gradient decreased from
41.2 ± 12.17 mmHg at baseline to 8.6 ± 3.59 mmHg at 30 days. The mean peak gradient decreased
from 73.2 ± 21.51 mmHg at baseline to 17.7 ± 7.30 mmHg at 30 days.
Results of SOURCE XT
A total of 2688 patients were enrolled. The vast majority of patients (96%) were treated with either the
transapical (TA) or transfemoral (TF) approach. Only a small proportion of patients were treated with
transaortic (TAo) or subclavian approaches. The implant approach was 62.7% for TF, 33.3% for TA,
3.76% for TAo and 0.3% for subclavian. The results only include the TF, TA and TAo approaches
(n = 2680).
Using K-M event rates at 30 days post implant for the TF, TA/TAo population, 6.2% of patients had
died, 3% due to a cardiac death, 3.6% of patients had suffered a stroke, and 6.6% had a major
vascular complication. Major/life threatening bleeding had occurred in 14.9% of patients, major
bleeding in 10.2%, and renal failure or AKI in 17.8%. Permanent pacemakers were implanted in 9.5%
of patients. Using K-M event rates at 1 year post implant for the TF, TA/TAo population, 19.5% of
patients had died, 9.5% of these from cardiac death, and 6.3% of patients had suffered a stroke.
Major/life-threatening bleeding had occurred in 17.3% of patients, major bleeding in 12%, major
vascular complications in 7.2%, renal failure or AKI in 20.5% and 11% of patients had a new
pacemaker implanted.
Of the 2688 patients that were enrolled, fifty-seven (57) of these patients had the SAPIEN XT valve
implanted into a failing surgical prosthesis. The TF approach was used in 23 patients, and the
TA/TAo approach was used in 34 patients. The implanted valve size was 23 mm in 38 patients
(66.7%), 26 mm in 14 patients (24.6%), and 29 mm in 5 patients (8.8%).
No deaths, no strokes, no major vascular complications, no life threatening bleedings, one (1) renal
failure, and no new permanent pacemakers were reported at 30 days post implant for the TF
population. At 1 year post implant, 3 deaths were reported for the TF population.
In the TA/TAo population, 3 deaths, 1 (major) stroke, 2 major vascular complications, 3 life
threatening bleedings, and 4 new permanent pacemakers were reported at 30 days. At 1 year post
implant, 4 additional deaths, 1 additional (minor) stroke, 1 additional major vascular complication, and
1 additional new permanent pacemaker were reported for the TA/TAo population.
The PARTNER II Cohort B Aortic Valve-in-Valve Registry (NR3/CANR3)
A clinical study was performed to establish a reasonable assurance of safety and effectiveness of
transcatheter aortic valve replacement with the Edwards SAPIEN XT valve in patients with a failing
surgical bioprosthetic aortic valve (i.e., “TAV-in-SAV”). The study was carried out as a single-arm
registry nested (i.e., the PARTNER II Trial), which was designated as “NR3.” NR3 was originally
approved for 100 patients and later expanded under a Continued Access Protocol (CAP). Data from
the original NR3 cohort and the NR3 CAP (CANR3) cohort were pooled at 30 days and 1 year data
was available for the NR3 cohort only.
Patients were treated at 40 investigational sites between June 12, 2012 and December 10, 2013. The
database for this PMA supplement reflected data collected through February 26, 2015 and included
199 patients (2 patients withdrew prior to treatment). By the last database extract performed on
February 26, 2015, all of these patients were included in the 30-day data analysis, and 97 patients
were included in the 1-year analysis.
The NR3 study was a single arm, prospective, observational, descriptive study without formal
hypothesis testing. The patients were limited to those who were deemed by a heart team to have a

15
mortality or major morbidity rate of ≥ 50% for replacement of a failing surgical aortic valve and met the
sizing requirements for the 23 mm or 26 mm SAPIEN XT valve. The specific sizing requirements
were imposed because the 29 mm SAPIEN XT valve was not available when the study was initiated.
Contractors were utilized for analysis and interpretation of the clinical data, including an independent
Data Safety Monitoring Board (DSMB) that was instructed to notify the applicant of any safety or
compliance issues, a Clinical Event Committee (CEC) that was responsible for adjudicating endpoint-
related events reported during the trial per definitions established a priori, an Electrocardiography
(ECG) Core Lab for independent analysis of rhythm and occurrence of myocardial infarction, and an
Echocardiography Core Lab for independent analysis of all echocardiograms.
Results of PARTNER II Cohort B Aortic Valve-in-Valve Registry (NR3/CANR3)
Since identical protocols were used in the pivotal and CAP cohort investigations, data from the two
cohorts were pooled.
The “Attempted Implant” population consisted of all screen success patients for whom the index
procedure was started. The “Valve Implant” population consisted of those patients for whom the valve
implant process was completed. A total of 199 patients were screened for study participation. Two
patients withdrew consent prior to treatment; therefore, there were 197 “Attempted Implant” patients.
Two “Attempted Implant” patients were excluded from the “Valve Implant” population, because in one
patient, intra-procedural TEE demonstrated a low transvalvular jet velocity (2.6 m/s) and gradient of
24 mmHg which did not meet the inclusion criteria, and in the other patient, the procedure was
aborted due to inability to place the purse string sutures for transapical access. The patient
disposition is summarized in Table 12.
The demographics of the pooled study population are summarized in the Table 13. The mean age
was 78.5 years, and 60.4% were male. A high proportion of patients had significant comorbidities,
frailty, and prior cardiac interventions. The mean STS score was 9.7, and 95.4% of all patients were
in NYHA classes III or IV.
Table 14 provides a summary of the failed surgical valves treated, which consisted of 94.4%
bioprosthesis, 4.6% homografts, and 1.0% other valve types. Aortic stenosis was the predominant
cause of prosthetic failure (54.2%), followed by mixed lesion (23.4%) and insufficiency/regurgitation
(22.4%).
The primary endpoint of all-cause mortality, all stroke, moderate or severe obstruction, or moderate or
severe paravalvular leak was 16.9% at 30 days and 38.0% at 1 year, as shown in Table 15.
No unanticipated adverse device effects (UADEs) were reported throughout the trial. Three explants
have been reported to date; one explant occurred at autopsy, and two during surgical aortic valve
replacement due to severe aortic insufficiency on postoperative day 5 and day 18, respectively. No
CEC adjudicated endocarditis was reported.
The key safety outcomes adjudicated by the CEC for this study are presented in Table 16 through
Table 18.
Valve hemodynamics as assessed by echocardiography is summarized in Table 19 and Figure 11
through Figure 15. The mean DVI increased from 0.27 ± 0.10 at baseline to 0.37 ± 0.09 at 30 days
and 0.39 ± 0.11 at 1 year. The mean gradient decreased from 36.1 ± 16.38 mmHg at baseline to 17.4
± 7.37 mmHg at 30 days, which was maintained at 1 year. The mean peak gradient decreased from
65.0 ± 26.76 mmHg at baseline to 32.7 ±
12.90 mmHg at 30 days, which was maintained at 1 year. Moderate/severe aortic regurgitation was
present in 43.7% of subjects at baseline, which decreased to 2.5% at 30 days and 1.9% at 1 year.
Moderate/severe paravalvular leak was present in 6.8% of subjects at baseline, 2.5% at 30 days, and
1.9% at 1 year.
It is important to note that although mean and peak gradients were significantly reduced as compared
to baseline for the “TAV-in-SAV” procedure, the residual mean and peak gradients were numerically
higher than those observed for TAVR procedures performed for native valve stenosis.

16
The NYHA class by visit is shown in Figure 16. About 89% of subjects were in NYHA I/II at 30 days
and 84% at
1 year as compared to 5% at baseline.
The mean improvement in 6MWD among the Attempted Implant population was 49.8 ± 169.9 meters
from baseline to 30 days and 86.1 ± 142.0 meters from baseline to 1 year.
The mean hospitalization stay among the Attempted Implant population was 7.9 ± 7.0 days, which
included 2.9 ± 5.0 days in the ICU.
The QoL at different time points as measured by the Kansas City Cardiomyopathy Questionnaire
(KCCQ) clinical summary score is shown in Figure 17. The mean KCCQ summary score among the
Attempted Implant population improved from 45.5 ± 21.8 at baseline to 68.0 ± 22.0 at 30 days and
70.4 at 1 year.
Device success was defined as successful vascular access, delivery and deployment and retrieval of
delivery system; correct positioning, intended performance (aortic valve area > 1.2 cm2and mean
aortic valve gradient < 20 mmHg or peak velocity < 3 m/s, without moderate or severe prosthetic
valve aortic regurgitation. It was achieved in 61.5 % of patients. In the vast majority of device failure
subjects, the failure was due to unintended performance of the valve; specifically, mean gradient ≥ 20
mmHg or peak velocity ≥ 3 m/s was observed in 62 cases and moderate/severe aortic regurgitation in
5 cases.

17
PARTNER II Cohort B Registries Clinical Data
Table 6:
Cohort B (Inoperable)
Baseline Characteristics and Echocardiographic Findings for NR1, NR4 and NR6 (AT Population)*
SAPIEN XT Valve (TA/TAo)
Characteristic
(N = 265)
Age - yr
82.0 ± 7.79
Male sex — no. (%)
141/265 (53.2%)
STS score†
10.3 ± 5.51
Logistic EuroSCORE‡
13.2 ± 11.96
NYHA class — no. (%):
I/II
24/264 (9.1%)
III/IV
240/264 (90.9%)
Coronary artery disease — no./total no. (%)
194/265 (73.2%)
Previous myocardial infarction — no./total no. (%)
56/265 (21.1%)
Previous intervention — no./total no. (%)
CABG
118/265 (44.5%)
PCI
107/265 (40.4%)
Balloon aortic valvuloplasty
72/265 (27.2%)
Peripheral vascular disease — no./total no. (%)
150/265 (56.6%)
COPD — no./total no. (%):
Any
101/265 (38.1%)
Oxygen-dependent
41/265 (15.5%)
Creatinine > 2 mg/dL (177 μmol/liter) — no./total no. (%)
28/265 (10.6%)
Atrial fibrillation — no./total no. (%)
95/265 (35.8%)
Permanent pacemaker — no./total no. (%)
43/265 (16.2%)
Pulmonary hypertension — no./total no. (%)
34/254 (13.4%)
Frailty§— no./total no. (%)
97/254 (38.2%)
Extensively calcified aorta — no./total no. (%)
42/254 (16.5%)
Chest-wall deformity — no./total no. (%)
6/254 (2.4%)
Liver disease — no./total no. (%)
9/265 (3.4%)
Echocardiographic findings
Aortic-valve area — cm2
0.7 ± 0.19
Mean aortic-valve gradient — mmHg
41.2 ± 12.17
Mean LVEF — %
52.5 ± 13.37
Moderate or severe mitral regurgitation** — no./total no. (%)
70/232 (30.2%)
* Plus–minus values are mean ± SD. To convert the value for creatinine to micromoles per liter, multiply by 88.4.
AT denotes As Treated population, CABG denotes coronary-artery bypass grafting, COPD chronic obstructive
pulmonary disease, LVEF left ventricular ejection fraction, NYHA New York Heart Association, PCI percutaneous
coronary intervention, and TAVR transcatheter aortic-valve implantation.
†The Society of Thoracic Surgeons (STS) score measures patient risk at the time of cardiovascular surgery on a
scale that ranges from 0% to 100%, with higher numbers indicating greater risk. An STS score higher than 10%
indicates very high surgical risk.
‡The logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE), which measures patient
risk at the time of cardiovascular surgery, is calculated with the use of a logistic-regression equation. Scores
range from 0% to 100%, with higher scores indicating greater risk. A logistic EuroSCORE higher than 20%
indicates very high surgical risk.
§Frailty was determined by the surgeons according to prespecified criteria.
** Moderate or severe mitral regurgitation was defined as regurgitation of grade 3+ or higher.

18
Table 7:
Cohort B (Inoperable) Clinical Outcomes at 30 days for NR1, NR4 and NR6
(AT Population)*
OutcomeaSAPIEN XT Valve
(N = 265)
Death from any cause
21/265 (7.9%)
Major Stroke
5/265 (1.9%)
Repeat hospitalizationb
8/265 (3.0%)
Death from any cause or major stroke or repeat hospitalization
31/265 (11.7%)
Myocardial Infarction
4/265 (1.5%)
Major Vascular Complications
15/265 (5.7%)
Renal Failurec
7/265 (2.6%)
Disabling Bleeding Eventd
30/265 (11.3%)
Cardiac Reinterventione
8/265 (3.0%)
Endocarditis
0/265 (0.0%)
New Atrial Fibrillationf
9/167 (5.4%)
New pacemaker
12/265 (4.5%)
* AT = As Treated, NA = not applicable, TAVR = transcatheter aortic valve replacement. Data presented as n/N
(%) of patients.
a. CEC adjudicated.
b. Repeat hospitalizations were included if they were due to aortic stenosis or complications of the valve
procedure (e.g., TAVR).
c. Renal failure is defined as stage III acute kidney injury: Increase in serum creatinine to ≥ 300% (3 x increase
compared with baseline) or serum creatinine of ≥ 4 mg/d (≥ 354 μmol/L) with an acute increase of at least 0.5
mg/dl (44 μmol/L).
d. Disabling bleeding: Fatal bleeding OR bleeding in a critical area or organ, such as intracranial, intraspinal,
intraocular, or pericardial necessitating pericardiocentesis, or intramuscular with compartment syndrome OR
bleeding causing hypovolemic shock or severe hypotension requiring vasopressors or surgery OR overt source
of bleeding with drop in hemoglobin of ≥ 5 g/dL or whole blood of packed red blood cells (RBC) transfusion ≥ 4
units.
e. Cardiac reintervention includes any intervention that repairs, alters or replaces a previously operated valve
OR balloon aortic valvuloplasty OR Surgical aortic valve replacement OR valve in valve
f. Based on 167 patients at 30 days.
Table 8:
Conduction Disturbance Requiring Pacemaker to 30 Days for NR1, NR4 and NR6 (CEC Adjudicated) (AT
Population)
SAPIEN XT Valve
(TA/TAo)
(N = 265)
Event Events
Patients with
Event
New Permanent Pacemaker- All Patients
1
0-30 Days
12
12/265 (4.5%)
New Permanent Pacemaker – Patients without pre-procedural pacemaker
2
0-30 Days
12
12/222 (5.4%)
1Subjects with pacemaker or ICD at baseline are included (all patients included in denominator).
2Subjects with pacemaker or ICD at baseline are excluded (patients with baseline pacemaker/ICD subtracted
from denominator).
Note: The patients who received a new pacemaker in both rows are the same patients. The only difference is the
denominators.

19
Figure 3:
All-Cause Mortality, Major Stroke or Re-Hospitalization to
30 Days, NR1, NR4, and NR6 – TA/TAo
(AT Population)
0%
10%
20%
30%
40%
50%
60%
70%
0 5 10 15 20 25 30
All-Cause Mortality or Major Stroke or
Rehospitalization
Days Post-Procedure
SAPIEN XT
11.8%
No. at Risk
SAPIEN XT
265
250
247
245
241
240
234

20
Figure 4:
All-Cause Mortality to 30 Days, NR1, NR4, and NR6 –
TA/TAo
0%
10%
20%
30%
40%
50%
60%
70%
0 5 10 15 20 25 30
All-Cause Mortality
Days Post-Procedure
SAPIEN XT
8.0%
No. at Risk
SAPIEN XT
265
254
251
250
248
247
241
Figure 5:
Major Stroke at 30 Days, NR1, NR4, and NR6 – TA/TAo
(AT Population)
0%
5%
10%
15%
20%
0 5 10 15 20 25 30
Major Stroke
Days Post-Procedure
SAPIEN XT
1.9%
No. at Risk
SAPIEN XT
265
250
247
247
246
245
239
Other manuals for SAPIEN XT
1
Table of contents
Other Edwards Medical Equipment manuals
Edwards
Edwards EV1000 Clinical Platform User manual
Edwards
Edwards EV1000 Clinical Platform User manual
Edwards
Edwards VolumeView EV1000 User manual
Edwards
Edwards SAPIEN 3 User manual
Edwards
Edwards CG16K User manual

Edwards
Edwards ClearSight System User manual

Edwards
Edwards APG200 User manual

Edwards
Edwards ES Series User manual

Edwards
Edwards EV1000A User manual
Edwards
Edwards Acumen IQ Cuff User manual