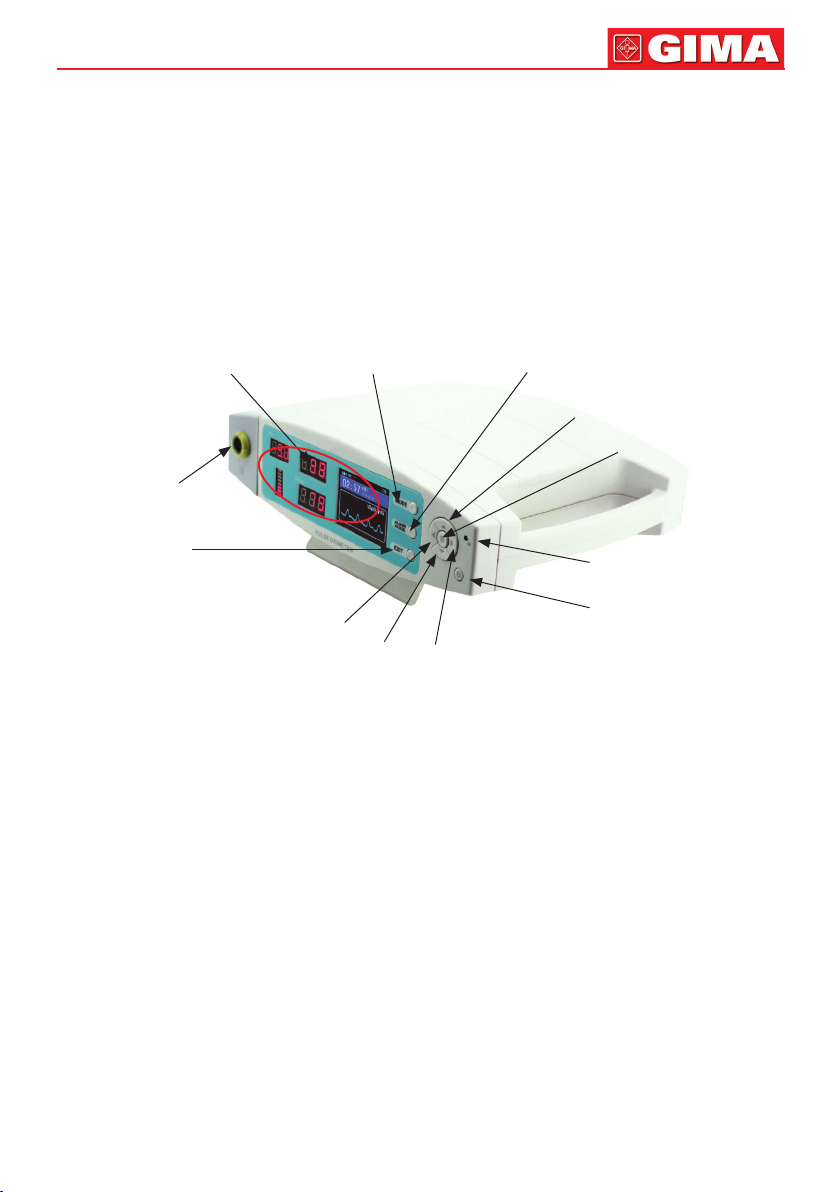
5
1.3 Attention
Keep the device away from dust, vibration, corrosive substances, tinder,
high temperature and moisture.
If the device gets wet, please stop operating it.
When it is carried from cold environment to warm or humid environment,
please do not use it immediately.
DO NOT operate keys on front panel with sharp materials.
High temperature or high pressure steam disinfection of the device is not
permitted. Refer to User Manual in the relative chapter (7.1) for instructions
of cleaning and disinfection.
DO NOT have the device immerged in liquid. When it needs cleaning, please
wipe its surface with medical alcohol by soft material. Do not spray any liq-
uid on the device directly.
When cleaning the device with water, the temperature should be lower than
60°C.
The fingers which are too thin or too cold may affect the measure accura-
cy, please clip the thicker finger such as thumb and middle finger deeply
enough into the probe.
The device can be used to adult and child. Whether the device is used to
adult or children, it depends on the probe selected.
The update period of data is less than 5 seconds, which is changeable ac-
cording to different individual pulse rate.
Please read the measure value when the waveform on screen is equably and
steady-going. This measure value is optimal value, and the waveform at the
moment is the standard one.
If some abnormal conditions appear on the screen during test process, pull
out the finger and reinsert to restore normal use.
The device has life for three years.
The device has alarm function, users can check on this function according
to chapter 6.1 as reference.
The device has the function of limit alarm. When the measure data is beyond
limit, the device would start to alarm automatically if the alarm function is
on.
The device has alarm function. This function can either be paused, or closed
for good. Please check the chapter 6.1 as reference.
The device may not work for all patients. If you are unable to achieve stable
readings, discontinue use.
1.4 EMC statement
Electromagnetic compatibility shall be considered during device in use, be-
cause high electromagnetic portable or mobile RF equipment will interfere the
working of the device.
Usage of other cables will affect the EMC performance of the device, please
use the standard accessories.