8
English
• Cochlear implants SYNCHRONY 2 Compressed are intended to be used in cochleae
with moderate obliteration, ossication, or malformation for an electrode insertion
depth of about 15mm as per request of the surgeon.
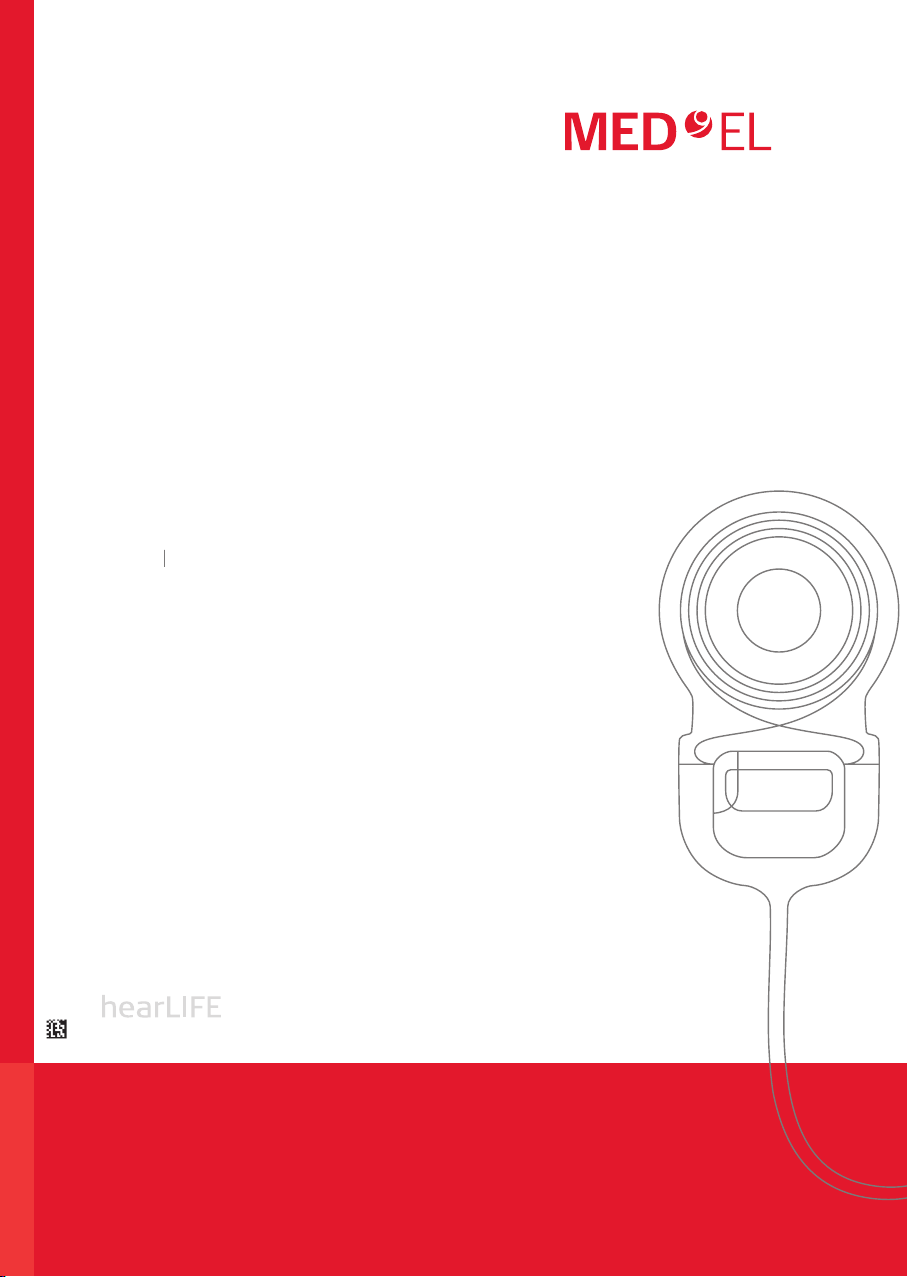
• Cochlear implants SYNCHRONY 2 FLEXSOFT are intended to be used in open cochleae
(no obliteration or ossication) for an electrode insertion depth of about 31mm.
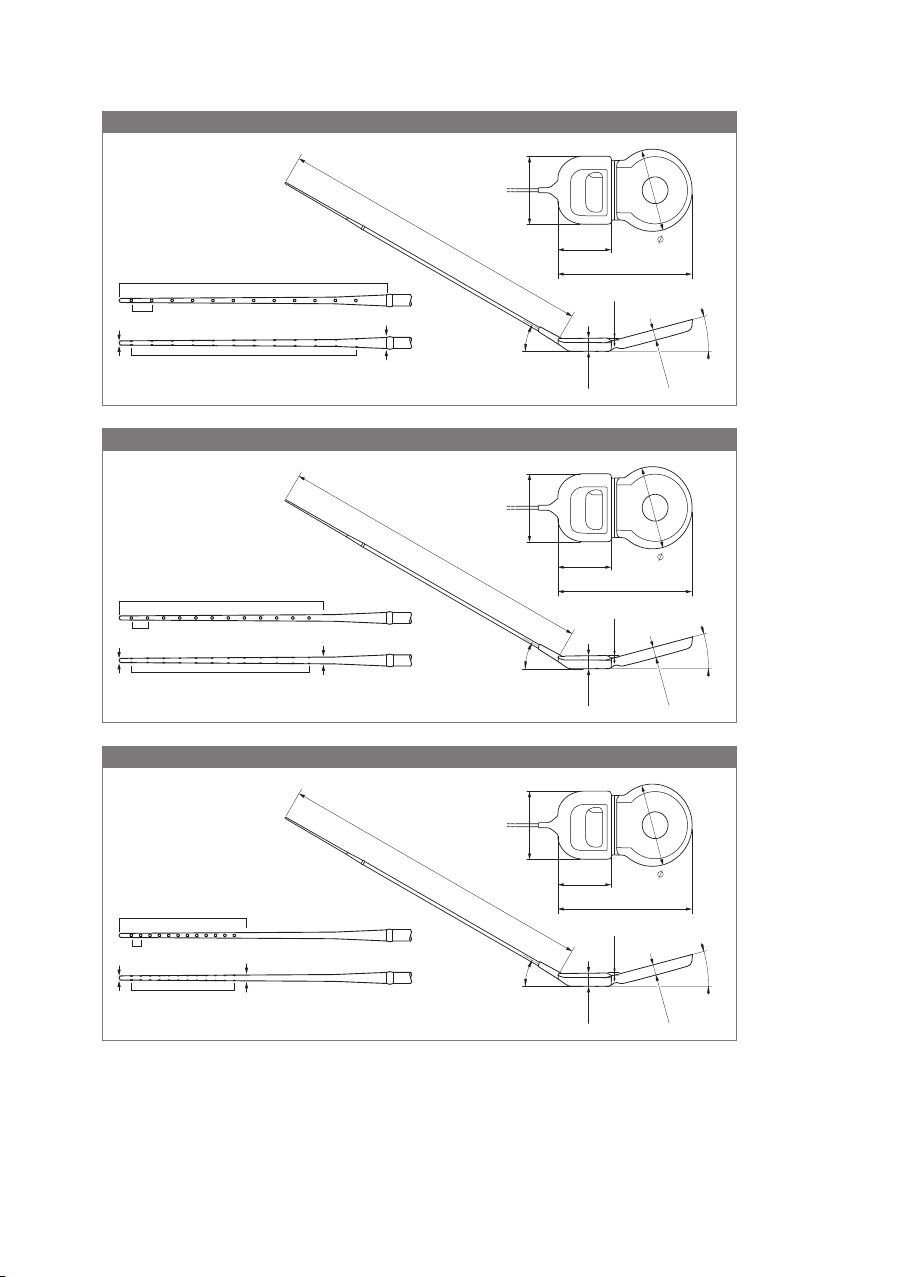
• Cochlear implants SYNCHRONY 2 FLEX28 are intended to be used in open cochleae
(no obliteration or ossication) for an electrode insertion depth of about 28mm.
• Cochlear implants SYNCHRONY 2 FLEX26 are intended to be used in open cochleae
(no obliteration or ossication) for an electrode insertion depth of about 26mm.
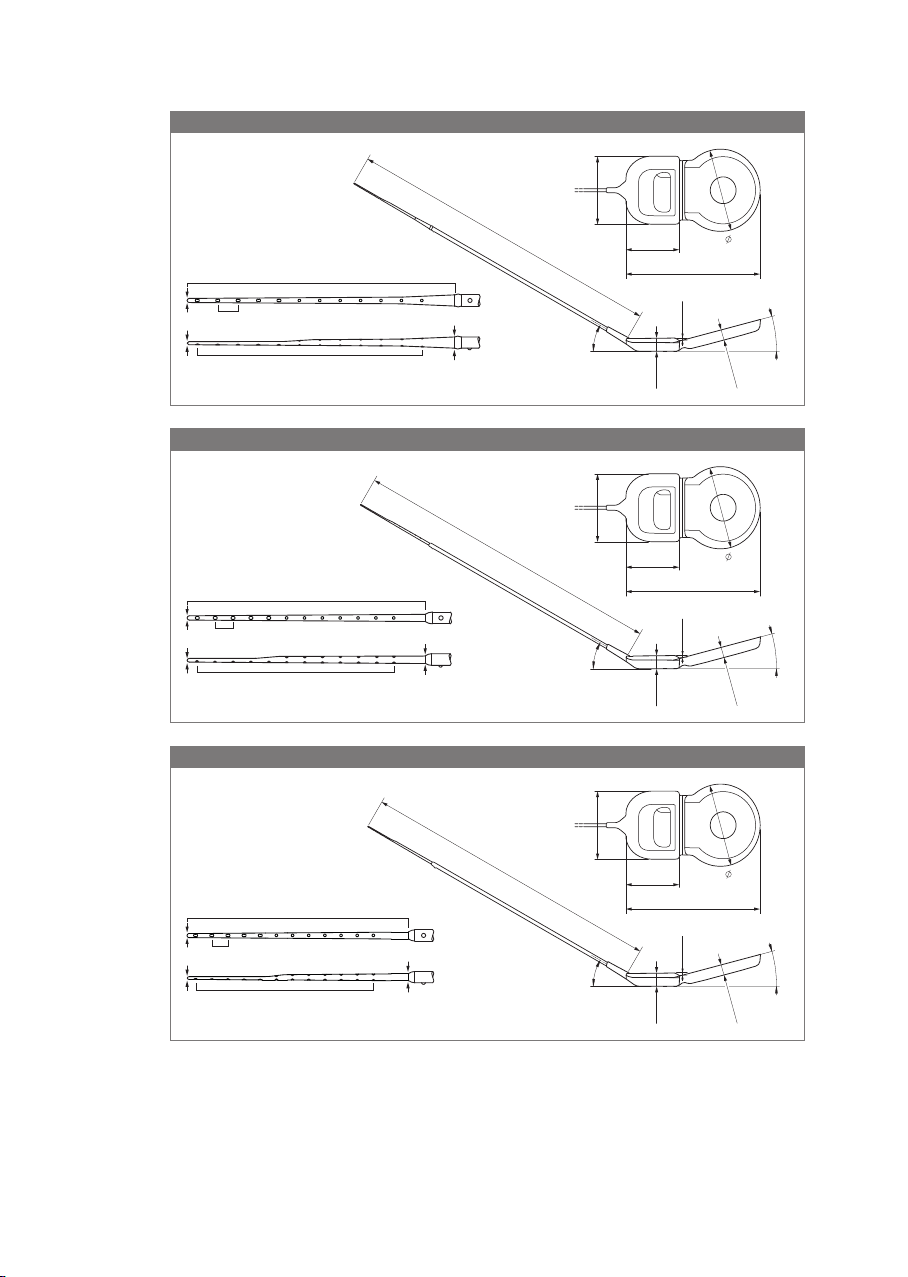
• Cochlear implants SYNCHRONY 2 FLEX24 for non‑EAS indication are intended to be
used in open cochleae (no obliteration or ossication) for an electrode insertion
depth of about 24mm as per request of the surgeon.
• Cochlear implants SYNCHRONY 2 FLEX24 used for EAS are indicated for partially deaf
individuals with mild to moderate sensorineural hearing loss in the low frequencies,
sloping to a profound sensorineural hearing loss in the high frequencies.
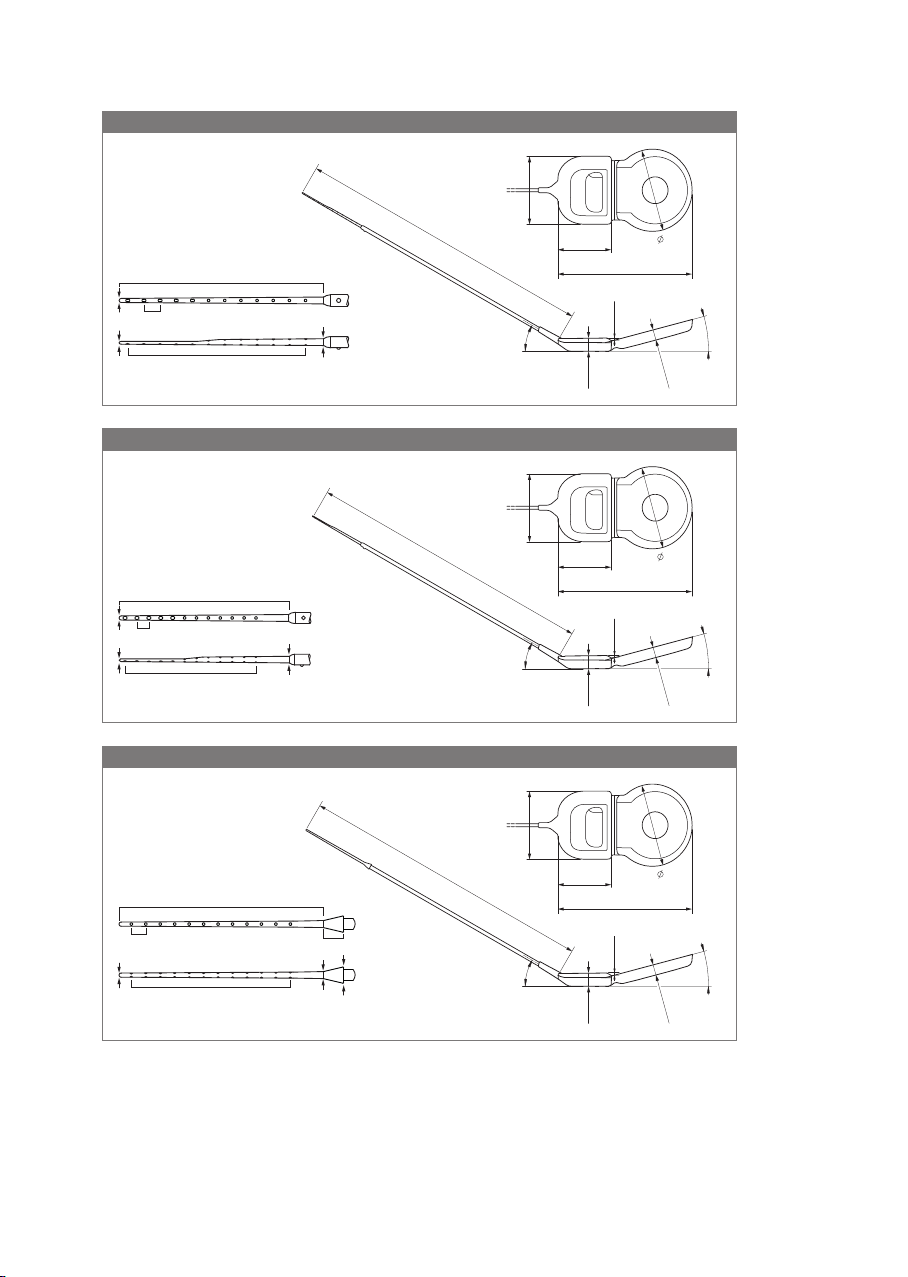
• Cochlear implants SYNCHRONY 2 FLEX20 for non‑EAS indication are intended to be
used in open cochleae (no obliteration or ossication) for an electrode insertion
depth of about 20mm as per request of the surgeon.
• Cochlear implants SYNCHRONY 2 FLEX20 used for EAS are indicated for partially deaf
individuals with mild to moderate sensorineural hearing loss in the low frequencies,
sloping to a profound sensorineural hearing loss in high frequencies.
• Cochlear implants SYNCHRONY 2 FORM24 are intended to be used in open cochleae
(no obliteration or ossication) or in cochleae with malformation for an electrode
insertion depth of about 24mm and/or when cerebrospinal uid (CSF) leakage is
expected.
• Cochlear implants SYNCHRONY 2 FORM19 are intended to be used in cochleae with
malformation, obliteration or ossication for an electrode insertion depth of about
19mm and/or when cerebrospinal uid (CSF) leakage is expected.
Contraindications
An individual must not be implanted,
• if the individual is known to be intolerant of the materials used in the implant
(including medical grade silicone, platinum, iridium and parylene c);
• if there is an absence of cochlear development;
• if the cause of deafness is non‑functionality of the auditory nerve and/or the upper
auditory pathway;
• if external or middle ear infections are present or if the tympanic membrane is
perforated in the ear to be implanted;
• if there are medical contraindications to surgery of the middle and inner ear and
anaesthesia as required;