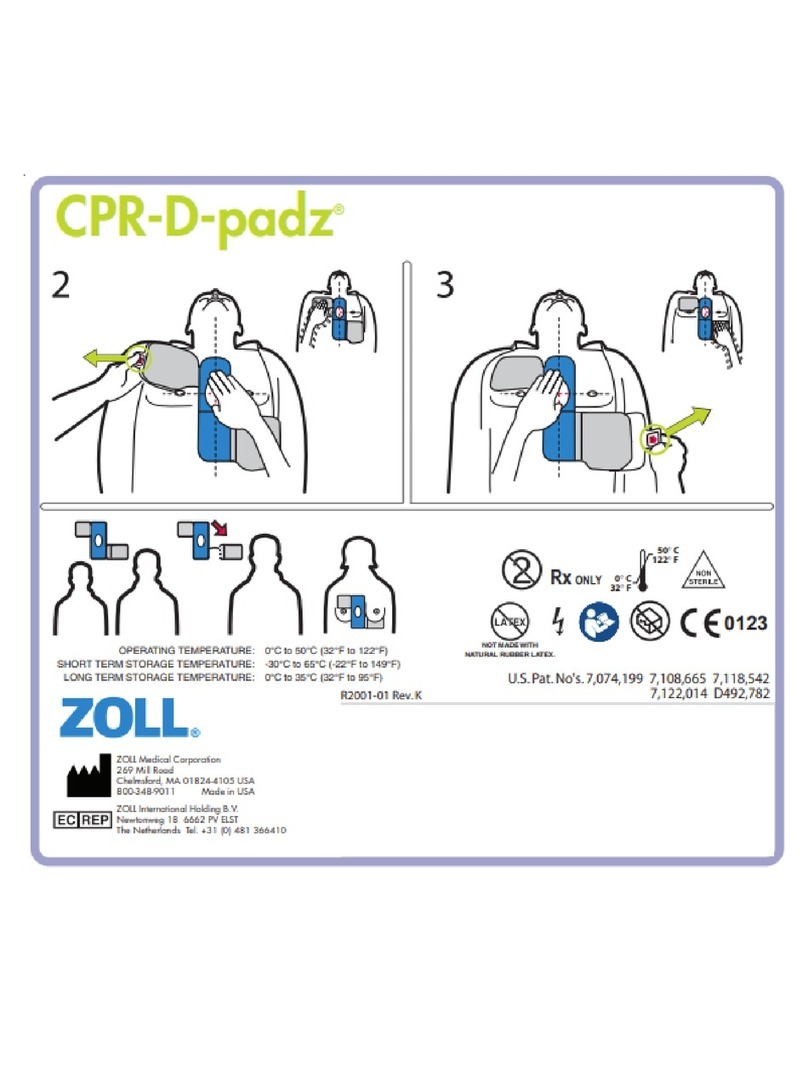
Cool Line®Intravascular Heat Exchange Catheter (Custom Luer)
Instructions for Use
CL-2295A/8700-0781-01
2 of 10
ZOLL
6.
Warnings and Precautions
WARNING: The catheter and Start-Up Kit could
potentially misconnect with other devices with small bore
connectors. Such misconnection errors could result in
patient injury or death.
CAUTION: The custom Luers on the catheter and SUK
may reduce the risk of misconnections but still have the
potential for misconnections with these specific medical
device applications: Breathing Systems & Driving Gases
applications, Enteral & Gastric applications, Urethral &
Urinary applications, Limb Cuff Inflation applications,
Neuraxial applications, and Intravascular or Hypodermic
applications. Always use caution when connecting ZOLL
catheters and SUK’s to these and other medical device
applications.
CAUTION: Ensure that the catheter and/or Start-Up Kit
are not connected to an IV or other medical device.
1. SINGLE USE ONLY. The product is designed for single
use only. Do not resterilize or reuse. Do not reinsert, once
removed from the patient. Do not alter the catheter in any
way. Maximum use period: 7 days.
2. The use of intravascular cooling devices controls fever,
including fever due to sepsis. Care must be taken to assess
patients for sepsis.
3. Alcohol and acetone can weaken the structure of the shaft
material. Care should thereforebetaken when infusingdrugs
containing alcohol or when using alcohol or acetone when
performing routine catheter care and maintenance. Alcohol
should not be used to declot the catheter.
4. Use of a syringe smaller than 10 ml to irrigate or declot an
occluded catheter may cause intraluminal leakage or
catheter rupture.
5. Caution: If blood is observed within the sterile saline
circuit, stop the procedure.
6. The catheter is coated with heparin. This may induce or
aggravate pre-existing heparin-induced thrombocytopenia
(HIT).
7. Central venous catheterization should only be performed
by well-trained personnel well-versed in anatomical
landmarks and safe technique. Personnel should also have
knowledge of potential complications.
8. The catheter should be placed via a jugular, subclavian, or
femoral vein approach only.
9. Do not allow the catheter to be placed into the right atrium
or right ventricle. If placed via the jugular or subclavian
veins, the catheter should be positioned so that the distal tip
of the catheter is in the superior vena cava above its junction
with the right atrium and parallel to the vessel wall. X-ray
examination should be used to ensure that the catheter is not
in the right atrium or ventricle. The distal tip of the catheter
should be positioned at a level above either the azygos vein
or the carina of the trachea, whichever is better visualized.
10. If placed via the femoral vein, the catheter should be
positioned so that its distal tip is in the inferior vena cava,
below its junction with the right atrium and parallel to the
vessel wall.
11. Possible complications with central venous catheters
include: atrial or ventricular perforation, cardiac
tamponade, air embolism, catheter embolism, thoracic
duct laceration, bacteremia, septicemia, thrombosis,
inadvertent arterial puncture, hematoma formation,
hemorrhage, nerve damage and dysrhythmias.
12. All Luer-Lock connections and covers must be securely
tightened to prevent air embolism or fluid or blood loss.
13. Never use excessive force in moving the catheter or
guidewire. If resistance is encountered, an x-ray should
be performed to identify the reason for the resistance.
14. Passage of the guidewire into the right heart can cause
dysrhythmias, right bundle branch block, vessel wall,
atrial or ventricular perforation.
15. Use only sterile normal saline for catheter priming. It is
the circulating fluid in the catheter.
16. The catheter should be routinely inspected for flow rate,
security of dressing, correct catheter position and for
secure Luer-Lock connections. Use the centimeter
markings to identify if the catheter position has changed.
17. Only x-ray examination can ensure that the catheter tip
has not entered the heart or no longer lies parallel to the
vessel wall. If the catheter position has changed, perform
an x-ray examination to confirm catheter tip position.
18. For blood sampling, temporarily shut off remaining
infusion ports through which solutions are being infused.
19. Use only a 30 cc or smaller syringe for blood sampling.
20. Use care when infusing drugs that may be affected by
cool temperatures (as low as 4ºC). Mannitol-containing
solutions are temperature sensitive and must not be
delivered through the catheter except for a rapid push of
up to a concentration of 20% mannitol solution, followed
by saline flush. Higher than a 20% concentration of
mannitol or drip or infusion pump delivery of mannitol
must be done via a separate line.
21. Do not infuse into the orange Luer-Lock connections.
22. Use only the ZOLL suture tab and clip provided in the kit
to prevent catheter damage.
23. Not intended for pediatric or neonatal use.
24. Cardiac Tamponade: Placement of indwelling catheters in
the right atrium is a practice that may lead to cardiac
perforation and tamponade. Practitioners placing central
venous catheters must be aware of this potentially fatal
complication before advancing the catheter too far relative to
patient size. The actual position of the tip of the indwelling
catheter should be confirmed by x-ray after insertion.
Central venous catheters should not be placed in the right
atrium unless specifically required for special relatively
short-term procedures, such as aspiration of air emboli
during neurosurgery. Such procedures are nevertheless risk
prone and should be closely monitored and controlled.
25. When connecting infusion sets/injection systems to
ZOLL catheters, do not exceed 100 psi/689 kPa.
WARNING: INTRALUMINAL LEAKAGE Intraluminal
leakage between the saline Luer and infusion Luers is an
uncommon but potential catheter failure. In the event of such
a failure, sterile saline from the cooling circuit will be
introduced into the patient. Intraluminal leakage will usually
be associated with a fluid loss alarm, which will stop the
system. ALWAYS INVESTIGATE FLUID LEVEL
ALARMS. The cooling circuit is a closed loop system –
usually fluid loss alarms indicate a breach somewhere in this
closed loop. With any fluid loss alarm, check both the
integrity of the catheter and the Start-Up Kit (see below).