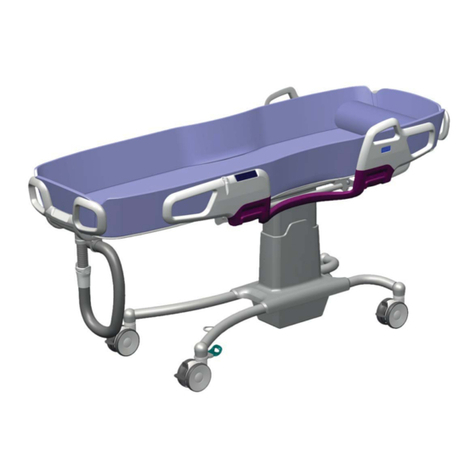
8
RISKS AND PRECAUTIONS
The following precautions should always be observed:
Side Rails and Restraints – WARNING: Use or non-use of restraints, including side rails,
can be critical to patient safety. Serious or fatal injury can result from the use (potential
entrapment) or non-use (potential patient falls) of side rails or other restraints. See related
Safety Information.
Patient Migration – Specialty surfaces have different shear and support characteristics
than conventional surfaces and may increase the risk of patient movement, sinking and /
or migration into hazardous positions of entrapment and / or inadvertent bed exit. Monitor
patients frequently to guard against patient entrapment.
Skeletal Traction or Unstable Fracture (if not contraindicated) - With skeletal traction,
unstable pelvic fracture or any other unstable fracture (to the extent not contraindicated),
maintain physician directed angle.
SAFETY INFORMATION
Bed Frame – Always use a standard healthcare bed frame with safeguards or protocols
that may be appropriate. It is recommended that Bed and Side Rails (if used) comply with
the Hospital Bed System Dimensional and Assessment Guidance To Reduce Entrapment.
Frame and Side Rails must be properly sized relative to the mattress to help minimize any
gaps that might entrap a patient’s head or body.
Turning – CAUTION: Prior to engaging turn feature, ensure that bed frame has side rails
and that all side rails are fully engaged in their full upright and locked position.
Brakes – Caster brakes should always be locked once the bed is in position. Verify wheels
are locked before any patient transfer to or from the bed.
Bed Height – To minimize risk of falls or injury, the bed should always be in the lowest
practical position when the patient is unattended.
Side Rails / Patient Restraints – Whether and how to use side rails or restraints is a
decision that should be based on each patient's needs and should be made by the patient
and the patient's family, physician and caregivers, with facility protocols in mind. Caregivers
should assess risks and benets of side rail / restraint use (including entrapment and
patient falls from bed) in conjunction with individual patient needs, and should discuss
use or non-use with patient and / or family. Consider not only the clinical and other needs
of the patient but also the risks of fatal or serious injury from falling out of bed and from
patient entrapment in or around the side rails, restraints or other accessories. In the
US, for a description of entrapment hazards, vulnerable patient prole and guidance
to further reduce entrapment risks, refer to FDA's Hospital Bed System Dimensional
and Assessment Guidance To Reduce Entrapment. Outside the US, consult the local
Competent Authority or Government Agency for Medical Device Safety for specic local
guidance. Consult a caregiver and carefully consider the use of bolsters, positioning aids
or oor pads, especially with confused, restless or agitated patients. It is recommended
that side rails (if used) be locked in the full upright position when the patient is unattended.
Make sure a capable patient knows how to get out of bed safely (and, if necessary, how
to release the side rails) in case of re or other emergency. Monitor patients frequently to
guard against patient entrapment.