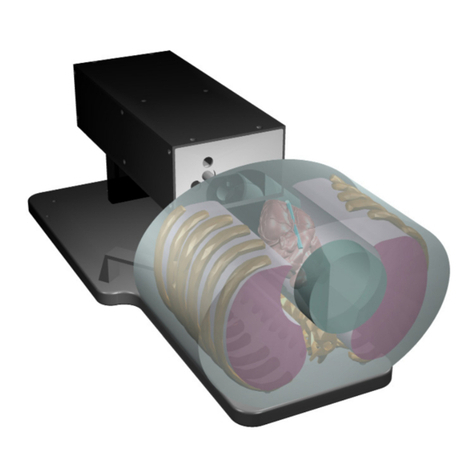
Cirs SHANE 13650001 User manual

1
900 Asbury Ave • Norfolk, Virginia 23513 • USA • Tel: 757-855-2765 • WWW.CIRSINC.COM
Phantom Patient for
VMAT-IMRT Verification
SHANE 13650001
SCAN
PLAN
LOCALIZE
TREAT
SHANE
SHOULDERS, HEAD AND NECK, END-TO-END

2
CONTENTS
Overview ........................................................................................... 3
Description of the Phantom ............................................................... 4
Specifications .................................................................................. 10
Use of the Phantom......................................................................... 11
Care & Handling .............................................................................. 14
Appendix......................................................................................... 14
Notes ............................................................................................ 18
Warranty.......................................................................................... 11

3
OVERVIEW
The CIRS Dose Verification Head and Shoulder Phantom is designed for end-
to-end testing of treatment planning systems from imaging acquisition through
dosimetry verification. The high fidelity anthropomorphic design contains complex
internal anatomy that provides a realistic clinical simulation to evaluate the chal-
lenging effects of intra- and extra-cranial anatomies. Head and shoulder portions
are manufactured as a single piece to enable use with various fixation devices. The
shoulder portion contains thoracic vertebras, which enables TPS verification to the
level of T2 vertebra. Shoulders also include electron density inserts for callibration.
Dosimetry measurements for treatment plan verification can be performed using
large Radiochromic or radiographic film positioned in the coronal plane of the phan-
tom and using ion chambers or other detectors, which can be positioned in four
parallel holes drilled through the phantom in Inferior-Superior direction.

4
DESCRIPTION OF THE PHANTOM
The phantom is based on the standard CIRS Model 038 Stereotactic End-to-End
Verification phantom (STEEV) and consists of two major components: head and
shoulders that are permanently attached. Head is cut axially at the vertex parallel to
the Frankfort horizontal plane. The rest of the phantom is cut coronally and serves
as a main dosimetry location. An ABS end plate is attached to the bottom of the
shoulders. It measures 2 cm in thickness.
Both vertex and shoulder end plate are at-
tached to the phantom using nylon screws.
Nylon thumb screws are used to disassem-
ble front and rear portions of the phantom.
An additional black nylon strap with a buckle
can be wrapped around the neck to avoid
deformation during storing, transportation
and phantom use.
Shoulders are reduced in size in superior-in-
ferior (SI) direction down to 7 cm to minimize
the weight of the phantom. The shoulders
have thoracic spine anatomy T1-T3 to extend
the useful dosimetry region.
The Phantom is drilled in four (4) locations to accommodate ion chambers or other
dosimetry detectors: one between the spine and trachea, and two (2) holes 3 cm
laterally from the central hole at the same coronal plane. These cavities can be used
for parotid and nasopharynx plan verification. With the spacer plugs provided, oro-
pharynx and hypopharynx plans can also be verified. A spinal hole is drilled centrally
in the spinal cord at the level of C2 vertebra for verification of treatment plan in the
neck. All holes measure 13 mm in diameter.

5
POSITIONING AND FILM REGISTRATION
The phantom contains unique features for proper and repeatable orientation, posi-
tioning and film-to-treatment plan registration. Laser alignment marks (crosshairs)
are aligned with an anterior hole and positioned on the vertex, front and sides of the
head and shoulders. Lateral and frontal marks identify the ISO center that is virtually
located at the level of nasopharynx in the SI direction. Lateral, Anterior and Posterior
laser marks on the head have four (4) ceramic (Al2O3) BBs Ø 1.5 mm at the center
for imaging plane identification.
There is one (1) solid rod with a single ceramic BB located only at the ISO imaging
plane, and there are four (4) solid rods for the IS holes with the same ceramic BBs,
located at the ISO imaging plane for ion chamber iso center localization. All rods
measure 330 mm in length.

6
The posterior portion of the phantom contains five (5) CT fiducial pins for film-to-
treatment plan registration. Four fiducials are located in an orthogonal configuration
and a fifth fiducial is 10 mm offset to avoid mistakes in film registration. They are
made of stainless steel and measure Ø 1 mm and 5 mm in length. The sharp tips
of the fiducials prick the film surface from behind making a precise point on the im-
age. See diagram at the bottom of this page for precise fiducial locations.
FILM DOSIMETRY
Film for dosimetry measurements can be received in a large coronal slice through
the head, neck and shoulders. Film slice is located approximately 10 mm from the
central axis of the anterior hole. There are five (5) additional CT fiducials pins for
image-to-treatment plan registration. Film can be cut to shape if desired before or
after placement using a sharp blade or scissors.

7
ION CHAMBER DOSIMETRY
The phantom is configured for use with PTW 31010 Semiflex 0.125 cc ion chamber
(CIRS cavity code CV511C), but can accommodate different ion chambers, diodes
or other detectors upon request.
To use an ion chamber through the IS holes in variable positions each
phantom is equipped with:
Ø 7 Spacer Plugs Breakdown: cavity plug to accommodate a tip of chamber
CV511C – 15 mm long - qty 1. Cylindrical plugs: 1 x 5 mm Long,1 x 10 mm Long,
2 x 20 mm Long, 4 x 50 mm Long, and 1 x 100 mm Long.
To use an Ion chamber at ISO location or at ISO+50 mm locations
phantom includes:
Plugs with BBs can be identified by externally engraved black rings marking the
location of the BB.
The various sets of sleeves and rods are color coded on the inferior end.
DESCRIPTION SIZE MATERIAL QTY
Sleeve CV511C 115 (L) mm Soft Tissue (STG) 3
Plug PL-CV511C 330 (L) mm Soft Tissue (STG) 1
Plug PL-CV511C with BB 330 (L) mm Soft Tissue (STG) 1
Ø 7mm Spacer Plugs CV511C Total Length 370 mm Soft Tissue (STG) 10
Ø 13mm Spacer Plugs CV511C Total Length 130 mm Soft Tissue (STG) 8
DESCRIPTION SIZE MATERIAL QTY
Cavity Rod CV511C@ISO 320 (L) mm Soft Tissue (STG) 1
Plug PL-CV511C 217(L) mm Soft Tissue (STG) 1
DESCRIPTION SIZE MATERIAL QTY
Cavity Rod CV511C@ISO+50 320 (L) mm Soft Tissue (STG) 1
Plug PL-CV511C 167(L) mm Soft Tissue (STG) 1
Ø 13 Spacer Plugs Breakdown: Cylindrical plugs: 2 x 5 mm Long, 2 x 10 mm Long,
2 x 20 mm Long, 2 x 30 mm Long.
Schematics For Ion Chamber Re-Positioning Using Spacer Plugs in 13 mm Ø
Schematics For Ion Chamber Re-Positioning Using Spacer Plugs Inside 7mm Ø Sleeves.

8
PATIENT POSITIONING MASKS.
CIRS has successfully
tested thermoplastic masks
from the following Manufac-
turers.
• Bionix,
• Klarity,
• Civco,
• Orfit
• Macromedics
DSPS (depicted)

9
Electron Density
To perform the electron density calibration the shoulders accommodate five (5)
permanent locations for electron density plugs: cortical bone, trabecular bone, lung
inhale, lung exhale and water. Plugs measure Ø 2.5 cm x 3 cm long. Bone plugs
are positioned at the approximately humerus locations. Lung plugs are located
closer to the center of the phantom on both sides, similar to the top of the lung
anatomy. A water vial with lid is positioned in the middle of the shoulders near the
trachea. The vial can be filled with distilled water or desired contrast agent. All plugs
are located to minimize imaging artifacts.

10
SPECIFICATIONS
Dimensions: 36 cm (W) x 36 cm (L) x 22cm (H)
Weight: 10.2 kg without rods
Materials: CIRS proprietary epoxy resins. See Appendix
The phantom includes:
Qty: Part No. (if app)
1 Head Vertex
1 Head & Shoulders Anterior part
1 Head & Shoulders Posterior part
1 End plate, ABS black
1 Black nylon strap with a buckle, used for holding head together
4 Solid Rod w/BB @ ISO center & @ + 50 mm, 330 mm long
3 Sleeve CV511C, 115 mm long
1 Plug PL-CV511C solid, 330 mm long
1 Plug PL-CV511C solid w/BB, 330 mm long
1 Plug PL-CV511C solid to fit Cavity Rod @ ISO center, 217 mm long
1Cavity Rod CV511C for Ion Chamber @ ISO location, 320 mm
1 Plug PL-CV511C solid to fit Cavity Rod @ ISO center + 50 mm, 167 mm long
1 Cavity Rod CV511C for Ion Chamber @ ISO+50 mm location, 320 mm
1 Spacer Plugs CV511C Kit, total length 370 mm x 7mm Ø (10 Pieces)
1 Spacer Plugs CV511C Kit, total length 130 mm x 13mm Ø (8 Pieces)
4 Spare Water vial (original 1 inside phantom)
1 Screw driver
6Spare set of Nylon screws ¼-20x1-1/4”(original 6 inside phantom)
6 Black nylon thumbscrews ¼-20x1/2”
4Spare set of Nylon pins 1/4x1” (original 4 inside phantom)
2 User’s Guide (Physical)
1 Packing List (physical)
1USB drive containing electronic user guide and packing list
1 Carry case, foam lined

11
USE OF THE PHANTOM
GENERAL INSTRUCTIONS
1. Carefully remove phantom from carry case.
2. Position the phantom face up on a clean, flat surface.
4.
5.
Using the screw driver provided, un-
screw the two nylon screws from the
vertex, starting with the lower screw.
3. Unbuckle the nylon strap.
Unscrew the four nylon screws from
the end plate.

12
6.
7.
Screw the temporary black nylon
thumbscrews to the anterior portion
of the phantom.
Lift anterior portion of the phantom
from the posterior end using black
thumbscrews. Carefully place on a
clean, flat surface.

13
8.
9.
10.
11.
12.
13.
14.
15.
1.
2.
3.
CT fiducials are sharp so they can secure the film. Use care during assembly,
disassembly and film placement to ensure you do not scratch yourself
or the film, or bend the fiducials.
Reassemble anterior and posterior portions of the phantom, matching pin-holes
for best fit. Push down to avoid an air gap between the phantom and film.
Screw the end plate and vertex back to place using nylon screws and screw
driver. Together with fiducials they will secure film.
Insert required solid rods with fiducials, CV511C ion chamber rod(s), or sleeve(s)
with spacer plugs.
Phantom is ready to image or treat with radiation.
Phantom can be originally imaged without film because the air gap between
anterior and posterior parts is minimized by centering conical head nylon screws.
Replace nylon strap around the neck.
Electron Density Measurements
Fill vial with water or contrast liquid
and close the lid.
Insert vial into the large central hole in
a shoulders, container end first.
Scan shoulders with a beam slice thru the mid place of the electron density plugs
and take ROI measurements.
Position film of the desired size
on the flat surface of the posterior
portion making sure it covers CT
fiducials.

14
CARE & HANDLING
Your phantom is manufactured from epoxy resin. Various other chemicals and fillers
have been added to the resin using proprietary CIRS tissue simulation technology.
See appendix for simulation data. As with most other epoxy plastics, your phantom
may discolor slightly over time. This process can be accelerated by direct exposure
to sunlight or extreme temperatures. It is recommended that when not in use, the
phantom be stored in a dark, fully climatized storage area.
Store and transport a phantom fully assembled and with a Black nylon strap around
the neck inside the carry case. Epoxy is quite durable, but can still be damaged if it
is dropped on a hard surface; so handle with care!
Most phantoms can be easily repaired. If damaged, contact CIRS. It is recommend-
ed that the phantom only be cleaned with mild detergent and water. Avoid solvent
based or abrasive cleaning agents. Engineers and physicists at CIRS are available
to answer specific questions regarding the materials, design and technical specifi-
cations of the phantom. Specific questions regarding quality assurance protocols
should be directed toward the appropriate equipment manufacturers and experts in
the field.
APPENDIX
ENERGY- MEV SPINAL CORD REFERENCE *1 SPINAL CORD, CIRS RATIO, %
0.04 0.2769 0.2768 99.96
0.06 0.2125 0.2124 99.95
0.08 0.1895 0.1894 99.95
0.10 0.1762 0.1761 99.94
0.20 0.1414 0.1413 99.93
0.40 0.1095 0.1095 100.00
0.60 0.0924 0.0924 100.00
0.80 0.0812 0.0811 99.88
1.00 0.0730 0.0729 99.86
2.00 0.0510 0.0510 100.00
4.00 0.0351 0.0350 99.72
6.00 0.0285 0.0285 100.00
8.00 0.0249 0.0249 100.00
10.0 0.0227 0.0227 100.00
20.0 0.0185 0.0185 100.00
30.0 0.0174 0.0174 100.00
El. Density, x1023, cm-3 3.449 3.488 99.97
Density, gcm-3 1.04 1.07 -
DENSITIES AND LINEAR ATTENUATION COEFFICIENTS IN CM-1 FOR TISSUE SUBSTITUTES
Reference:
1. ICRU Report 46, Photon, Electron, Proton and Neutron Interaction Data for Body Tissues (1992).
2. ICRP 23, Report of the Task Group on Reference Man (1975).
3. Electron Density of Water: 3.340 x 10^23 /cc

15
ENERGY- MEV AVERAGE SOFT TISSUE REFERENCE *2 AVERAGE SOFT TISSUE, CIRS RATIO, %
0.04 0.2679 0.2678 99.96
0.06 0.2087 0.2091 100.19
0.08 0.1871 0.1876 100.27
0.10 0.1742 0.1748 100.34
0.20 0.1401 0.1406 100.36
0.40 0.1086 0.1090 100.37
0.60 0.0917 0.0920 100.33
0.80 0.0805 0.0808 100.37
1.00 0.0724 0.0726 100.28
2.00 0.0505 0.0507 100.40
4.00 0.0347 0.0348 100.29
6.00 0.0282 0.0282 100.00
8.00 0.0247 0.0247 100.00
10.0 0.0225 0.0225 100.00
20.0 0.0182 0.0181 99.45
30.0 0.0171 0.0170 99.42
El. Density, x1023, cm-3 3.421 3.434 100.38
Density, gcm-3 1.03 1.055 -
ENERGY- MEV TRABECULAR BONE REFERENCE *1 TRABECULAR BONE, CIRS RATIO, %
0.04 0.4546 0.04536 99.8
0.06 0.2802 0.2806 100.1
0.08 0.2296 0.2303 100.3
0.10 0.2058 0.2065 100.3
0.20 0.1588 0.1596 100.5
0.40 0.1223 0.1229 100.5
0.60 0.1031 0.1036 100.5
0.80 0.0905 0.0909 100.4
1.00 0.0813 0.0817 100.5
2.00 0.0568 0.0571 100.5
4.00 0.0393 0.0395 100.5
6.00 0.0322 0.0323 100.3
8.00 0.0284 0.0284 100.00
10.0 0.0260 0.0260 100.00
20.0 0.0216 0.0215 99.5
30.0 0.0206 0.0205 99.5
El. Density, x1023, cm-3 3.844 3.863 100.5
Density, gcm-3 1.18 1.20 -
APPENDIX (CONT.)

16
ENERGY- MEV CORTICAL BONE REFERENCE *1 CORTICAL BONE, CIRS RATIO, %
0.04 1.2783 1.2693 99.3
0.06 0.6046 0.6025 99.7
0.08 0.4282 0.4273 99.8
0.10 0.3561 0.3560 100.0
0.20 0.2517 0.2513 99.8
0.40 0.1903 0.1903 100.0
0.60 0.1600 0.1601 100.1
0.80 0.1403 0.1404 100.1
1.00 0.1260 0.1261 100.1
2.00 0.0884 0.0885 100.1
4.00 0.0626 0.0624 99.7
6.00 0.0525 0.0523 99.6
8.00 0.0473 0.0471 99.6
10.0 0.0444 0.0441 99.3
20.0 0.0397 0.0391 98.5
30.0 0.0394 0.0387 98.2
El. Density, x1023, cm-3 5.952 5.956 100.1
Density, gcm-3 1.92 1.93 -
ENERGY- MEV AVERAGE BRAIN REFERENCE *1 AVERAGE BRAIN BRDT, CIRS RATIO, %
0.04 0.2791 0.2791 100.00
0.06 0.2135 0.2138 100.14
0.08 0.1902 0.1907 100.26
0.10 0.1767 0.1772 100.28
0.20 0.1418 0.1422 100.28
0.40 0.1098 0.1102 100.36
0.60 0.0927 0.0930 100.32
0.80 0.0814 0.0817 100.37
1.00 0.0731 0.0734 100.41
2.00 0.0511 0.0513 100.39
4.00 0.0352 0.0352 100.00
6.00 0.0286 0.0286 100.00
8.00 0.0251 0.0250 99.60
10.0 0.0229 0.0228 99.56
20.0 0.0186 0.0185 99.46
30.0 .0.176 0.0174 98.86
El. Density, x1023, cm-3 3.458 3.470 100.35
Density, gcm-3 1.04 1.069 -

17
ENERGY- MEV AVERAGE LUNG INHALE REFERENCE *1 AVERAGE LUNG INHALE, CIRS
LAA
RATIO, %
0.04 0.0537 0.0524 97.6
0.06 0.041 0.0411 100.2
0.08 0.0365 0.0367 100.6
0.10 0.0339 0.0341 100.6
0.20 0.0272 0.0274 100.7
0.40 0.0211 0.0212 100.5
0.60 0.0178 0.0179 100.6
0.80 0.0156 0.0157 100.6
1.00 0.014 0.0141 100.7
2.00 0.0098 0.0099 101
4.00 0.0068 0.0068 100
6.00 0.0055 0.0055 100
8.00 0.0048 0.0048 100
10.0 0.0044 0.0043 97.7
20.0 0.0036 0.0035 97.2
30.0 0.0034 0.0032 94.1
El. Density, x1023, cm-3 0.663 0.668 100.8
Density, gcm-3 0.2 0.205 -
ENERGY- MEV AVERAGE LUNG EXHALE REFERENCE *1 AVERAGE LUNG EXHALE, CIRS LH RATIO, %
0.04 0.1342 0.1313 97.8
0.06 0.1025 0.1012 98.7
0.08 0.0912 0.0904 99.1
0.10 0.0848 0.084 99.1
0.20 0.068 0.0675 99.3
0.40 0.0526 0.0523 99.4
0.60 0.0444 0.0442 99.5
0.80 0.039 0.0388 99.5
1.00 0.0351 0.0349 99.4
2.00 0.0245 0.0243 99.2
4.00 0.0169 0.0167 98.8
6.00 0.0137 0.0135 98.5
8.00 0.012 0.0117 97.5
10.0 0.011 0.0106 96.4
20.0 0.009 0.0085 94.4
30.0 0.0085 0.0079 92.9
El. Density, x1023, cm-3 1.658 1.648 99.4
Density, gcm-3 0.5 0.5 -
APPENDIX (CONT.)

18
NOTES:

19
CIRS
Receiving
900 Asbury Ave,
Norfolk, Virginia, 23513 USA
PRODUCT WARRANTY PERIOD
Model1365-00 60 months
WARRANTY
All standard CIRS products and accessories are warranted by CIRS against defects
in material and workmanship for a period as specified below. During the warranty
period, the manufacturer will repair or, at its option, replace, at no charge, a product
containing such defect provided it is returned, transportation prepaid, to the manu-
facturer. Products repaired in warranty will be returned transportation prepaid.
There are no warranties, expressed or implied, including without limitation any im-
plied warranty of merchantability or fitness, which extend beyond the description on
the face hereof. This expressed warranty excludes coverage of, and does not pro-
vide relief for, incidental or consequential damages of any kind or nature, including
but not limited to loss of use, loss of sales or inconvenience. The exclusive remedy
of the purchaser is limited to repair, recalibration, or replacement of the product at
manufacturer’s option.
This warranty does not apply if the product, as determined by the manufacturer, is
defective because of normal wear, accident, misuse, or modification.
NON-WARRANTY SERVICE
If repairs or replacement not covered by this warranty are required, a repair estimate
will be submitted for approval before proceeding with said repair or replacement
RETURNS
If you are not satisfied with your purchase for any reason, please contact your local
distributor prior to returning the product. Visit https://www.cirsinc.com/distributors/
to find your local distributor. If you purchased your product direct through CIRS,
call Customer Service at 800-617-1177, email [email protected], or fax an RMA
request form to 757-857-0523. CIRS staff will attempt to remedy the issue via
phone or email as soon as possible. If unable to correct the problem, a return
material authorization (RMA) number will be issued. Non-standard or “customized”
products may not be returned for refund or exchange unless such product is
deemed by CIRS not to comply with documented order specifications. You must
return the product to CIRS within 30 calendar days of the issuance of the RMA. All
returns should be packed in the original cases and or packaging and must include
any accessories, manuals and documentation that shipped with the product. The
RMA number must be clearly indicated on the outside of each returned package.
CIRS recommends that you use a carrier that offers shipment tracking for all returns
and insure the full value of your package so that you are completely protected if the
shipment is lost or damaged in transit. If you choose not to use a carrier that offers
tracking or insure the product, you will be responsible for any loss or damage to the
product during shipping. CIRS will not be responsible for lost or damaged return
shipments. Return freight and insurance is to be pre-paid.
WITH RMA NUMBER, ITEMS MAY BE RETURNED TO:
NOTES:

20
©
2015 Computerized Imaging Reference Systems Inc. All rights reserved.
Specifications subject to change without notice.
Publication: SHANE1365-00 UG 072220 _rev 5
Computerized Imaging Reference Systems, Inc. has
been certified by UL DQS Inc. to (ISO) 13485:2016.
Certificate Registration No.10000905-MP2016.
COMPUTERIZED IMAGING
REFERENCE SYSTEMS, INC.
900 Asbury Ave
Norfolk, Virginia 23513 USA
Toll Free: 800.617.1177
Tel: 757.855.2765
Fax: 757.857.0523
Email [email protected]
www.cirsinc.com
Technical Assistance
1.800.617.1177
Table of contents
Other Cirs Medical Equipment manuals

Cirs
Cirs MRIdian Phantom User manual
Cirs
Cirs Brachytherapy QA Phantom User manual

Cirs
Cirs 604-GS User manual
Cirs
Cirs 034 User manual

Cirs
Cirs 015DM User manual
Cirs
Cirs Dynamic Phantoms 008A User manual

Cirs
Cirs 057A User manual

Cirs
Cirs ZERDINE 049 User manual

Cirs
Cirs 715 User manual

Cirs
Cirs ATS539 User manual
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual