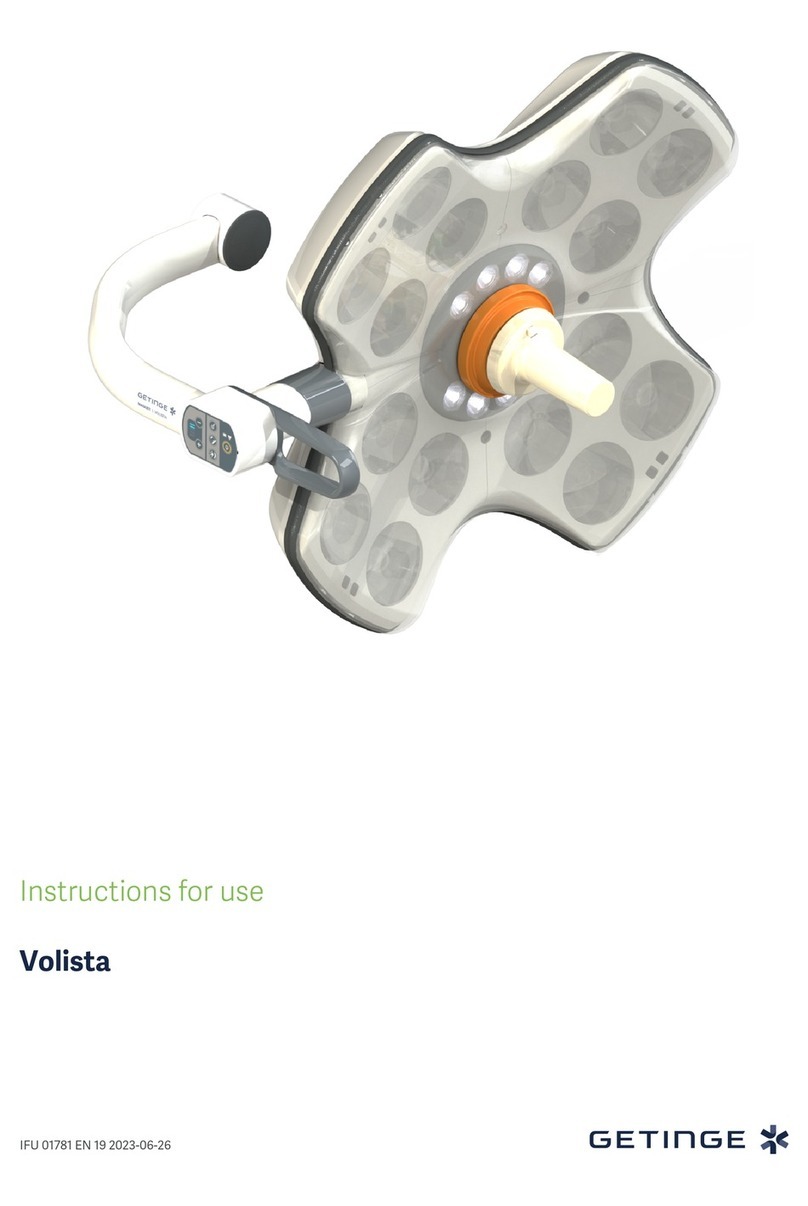
4 0113103 Notice d'utilisation / User manual / Benutzerhandbuch
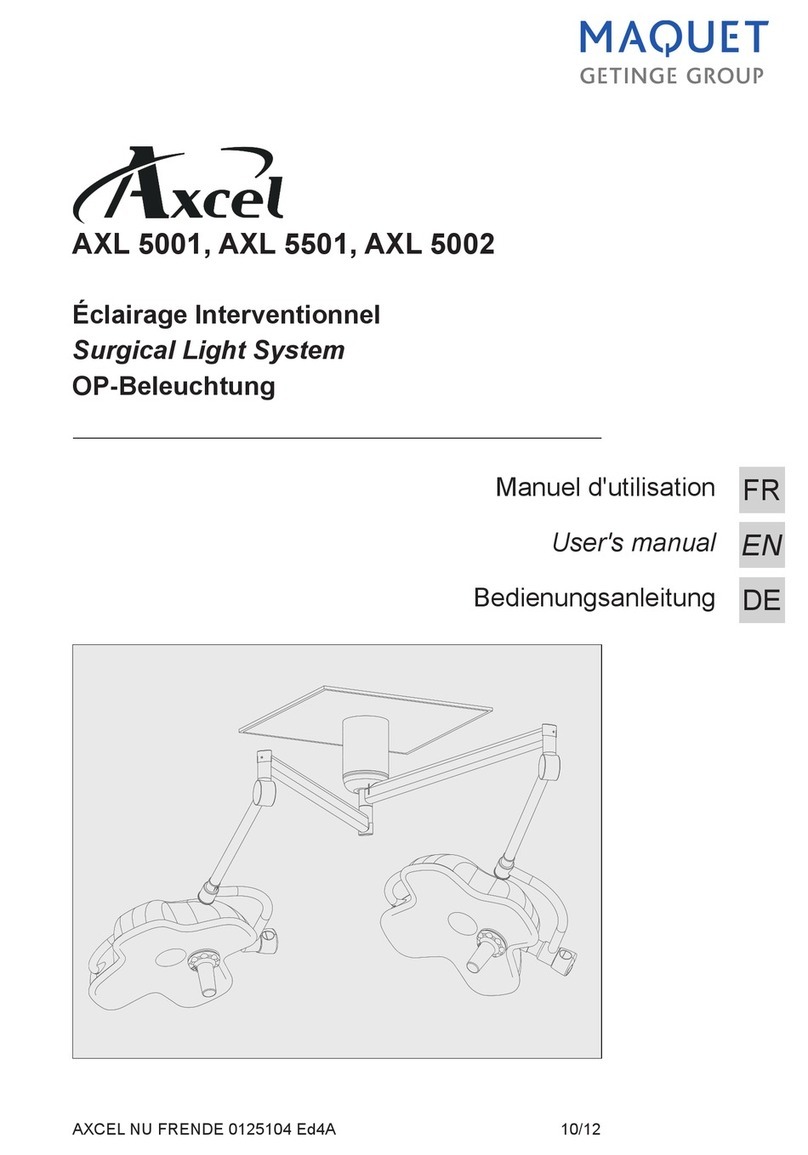
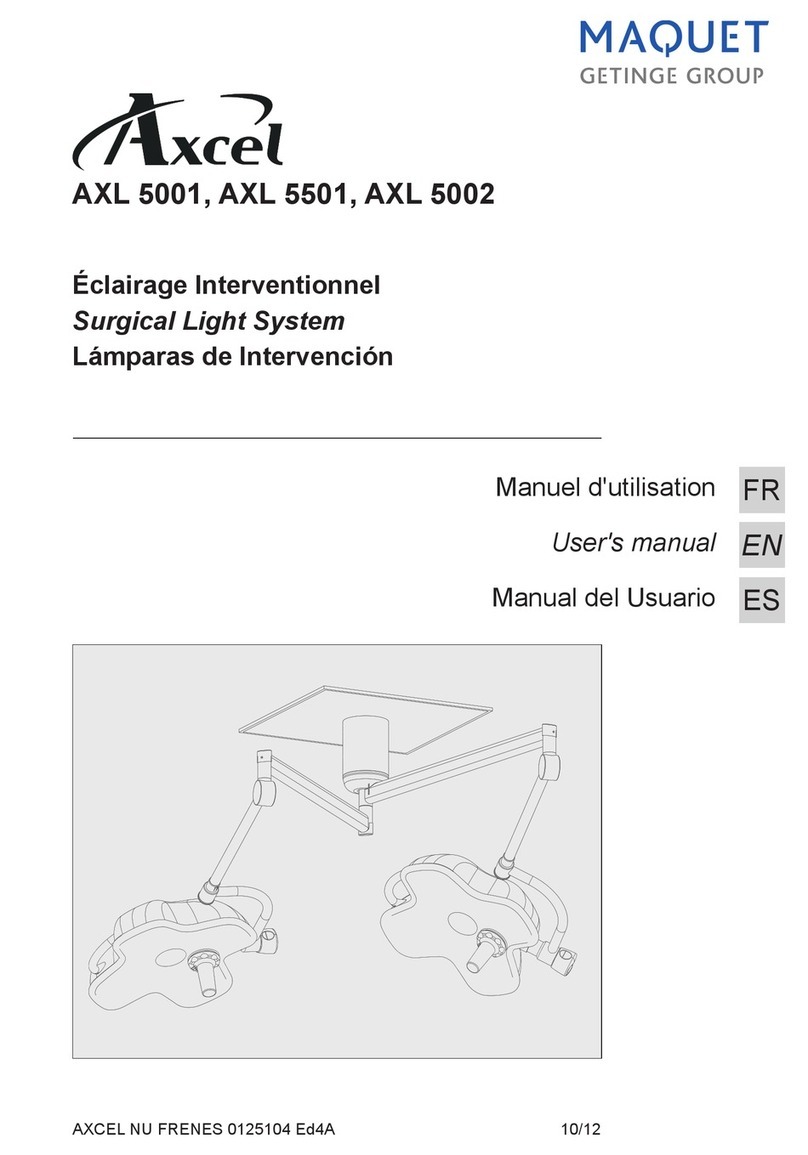
Éclairage opératoire
Surgical light
OP-Leuchten
QUALITY COMPLIENCE
Certication of MAQUET SA quality system.
LNE/G-MED certies that the quality system
created by Maquet SA for the design,
manufacturing, marketing, installation and
customer servicing of its surgical lights meets
the requirements of the following international
standards:
- ISO 9001:2000
- ISO 13485:2004
PRISMALIXTM is designed to fullfill the
following applicable standards:
• EN ISO 14971:2000
Medical devices - Application of risk
management to medical devices
(ISO 14971:2000)
•ENISO14971:2000/A1:2003
•EN60601-1:1990
Medical electrical equipment -
Part 1: General requirements for safety
Amendment A1:1993 to EN 60601-1:1990
Amendment A2:1995 to EN 60601-1:1990
Amendment A13:1996 to EN 60601-1:1990
•EN60601-1-2:2001
Medical electrical equipment -
Part 1-2: General requirements for safety
- Collateral standard: Electromagnetic
compatibility - Requirements and tests
•EN60601-1-4:1996
Medical electrical equipment -
Part 1-4: General requirements for safety -
Collateral standard: Programmable electrical
medical systems
Amendment A1:1999 to EN 60601-1-4:1996
•EN60601-1-6:2004
Medical electrical equipment -
Part 1-6: General requirements for safety -
Collateral standard: Usability
•EN60601-2-41:2000
Medical electrical equipment -
Part 2-41: Particular requirements for the
safety of surgical luminaires and luminaires
for diagnosis
This product was investigated according
to the following additional standards:
CAN/CSA-C22.2 No. 601.1-M90 (R2005)
(includes National Differences for Canada),
EN 60601-1:1990 + A1:1993 + A2:1995 +
A13:1996, UL 60601-1, 1st Edition, 2006-04-26
(includes National Differences for USA).
CE Marking/intended use
Compliance with the requirements of Directive
93/42/EEC dated of June 14th 1993, relating
to medical devices has been assessed in
accordance with Annex VII of this Directive. This
PRISMALIXTM Surgical lights range is a class I
device in accordance with Annex IX of Directive
93/42/EEC.
QUALITÄTSANFORDERUNGEN
Zertizierung des QM-Systems von MAQUET
SA.
LNE/G-MED bescheinigt, dass das von MA-
QUET SA für Entwicklung, Fertigung, Vertrieb,
Installation und Kundenservice eingerich-
tete Qualitätssicherungssystem für die OP-
Beleuchtungen die folgenden internationalen
Standards erfüllt:
- ISO 9001, Version 2000
- NF EN ISO 13485, Version 2004
Die Beleuchtung PRISMALIXTM wurde in
Übereinstimmung mit den Normen:
• EN ISO 14971:2000
Medical devices - Application of risk
management to medical devices
(ISO 14971:2000)
•ENISO14971:2000/A1:2003
•EN60601-1:1990
Medical electrical equipment -
Part 1: General requirements for safety
Amendment A1:1993 to EN 60601-1:1990
Amendment A2:1995 to EN 60601-1:1990
Amendment A13:1996 to EN 60601-1:1990
•EN60601-1-2:2001
Medical electrical equipment -
Part 1-2: General requirements for safety
- Collateral standard: Electromagnetic
compatibility - Requirements and tests
•EN60601-1-4:1996
Medical electrical equipment -
Part 1-4: General requirements for safety -
Collateral standard: Programmable electrical
medical systems
Amendment A1:1999 to EN 60601-1-4:1996
•EN60601-1-6:2004
Medical electrical equipment -
Part 1-6: General requirements for safety -
Collateral standard: Usability
•EN60601-2-41:2000
Medical electrical equipment -
Part 2-41: Particular requirements for the
safety of surgical luminaires and luminaires
for diagnosis
Dieses Produkt erfüllt folgende zusätzliche
Standards: CAN/CSA-C22.2 Nr. 601.1-M90
(R2005) (einschließlich Landesabweichungen
für Kanada), EN 60601-1:1990 + A1:1993
+ A2:1995 + A13:1996, UL 60601-1,
1. Edition, 2006-04-26 (einschließlich
Landesabweichungen für die USA).
MarkierungCE/vorgeseheneNutzung
Die Konformität mit den Bestimmungen der
europäischen Richtlinie 93/42/EWG des 14,Juni
1993 für medizinische Geräte wurde gemäß
Anhang VII überprüft und bestätigt. Dieser OP-
Beleuchtungen PRISMALIXTM gehört Klasse I
gemäß Anhang IX der Richtlinie 93/42/EWG.
EXIGENCES QUALITE
Certication du système qualité de l’entreprise
MAQUET SA.
Le LNE/G-MED certifie que le système qualité
développé par MAQUET SA pour la concep-
tion, la réalisation, la vente, l’installation et le
service après-vente d’éclairages opératoires
est conforme aux exigences des normes inter-
nationales :
- ISO 9001 version 2000
- NF EN ISO 13485 version 2004
L’éclairage opératoire PRISMALIXTM a été
conçu pour répondre aux normes suivantes :
• EN ISO 14971:2000
Medical devices - Application of risk
management to medical devices
(ISO 14971:2000)
•ENISO14971:2000/A1:2003
•EN60601-1:1990
Medical electrical equipment -
Part 1: General requirements for safety
Amendment A1:1993 to EN 60601-1:1990
Amendment A2:1995 to EN 60601-1:1990
Amendment A13:1996 to EN 60601-1:1990
•EN60601-1-2:2001
Medical electrical equipment -
Part 1-2: General requirements for safety
- Collateral standard: Electromagnetic
compatibility - Requirements and tests
•EN60601-1-4:1996
Medical electrical equipment -
Part 1-4: General requirements for safety -
Collateral standard: Programmable electrical
medical systems
Amendment A1:1999 to EN 60601-1-4:1996
•EN60601-1-6:2004
Medical electrical equipment -
Part 1-6: General requirements for safety -
Collateral standard: Usability
•EN60601-2-41:2000
Medical electrical equipment -
Part 2-41: Particular requirements for the
safety of surgical luminaires and luminaires
for diagnosis
Ce produit a fait l’objet de vérifications
conformément aux normes complémen-
taires suivantes : CAN/CSA-C22.2 No.
601.1-M90 (R2005) (comprend les différences
nationales pour le Canada), EN 60601-1:1990
+ A1:1993 + A2:1995 + A13:1996, UL 60601-1,
1ère édition, 2006-04-26 (comprend les diffé-
rences nationales pour les Etats-Unis).
MarquageCE/usageprévu
La conformité aux exigences de la Directive
93/42/CEE du 14 juin 1993relative aux dispositifs
médicaux a été évaluée selon l'Annexe VII de
la Directive. La gamme d’éclairage opératoire
PRISMALIXTM appartient à la Classe I selon
l'Annexe IX de la Directive 93/42/CEE.
Andegarantirtouteslesqualités
de nos produits, il est néces-
saire de prévenir MAQUET SA
en cas de changement d'environne-
ment ou d'utilisation.
In order to guarantee the quality
of our products, it is necessary to
contact MAQUET SA in case of use
or environment change.
Um die optimale Qualität unserer
Produkte zu gewährleisten,
müssenSieMAQUETSAüber jeden
Wechsel der Umgebung oder der
Verwendung informieren.