2
ENGLISH
Weight Capacity: 75kgs
INTENDED USE:
Longway Medical Accessories are Medical Devices intended to
compensate for a disability or inability of the bedridden user.
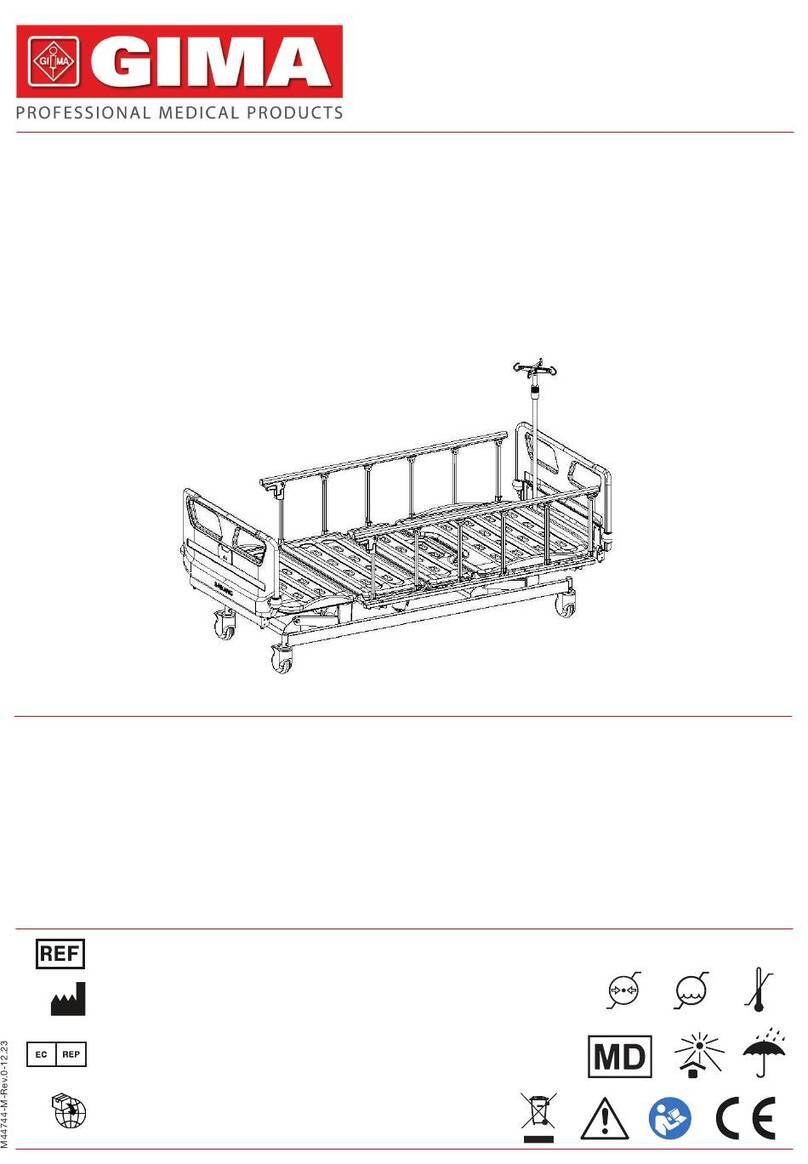
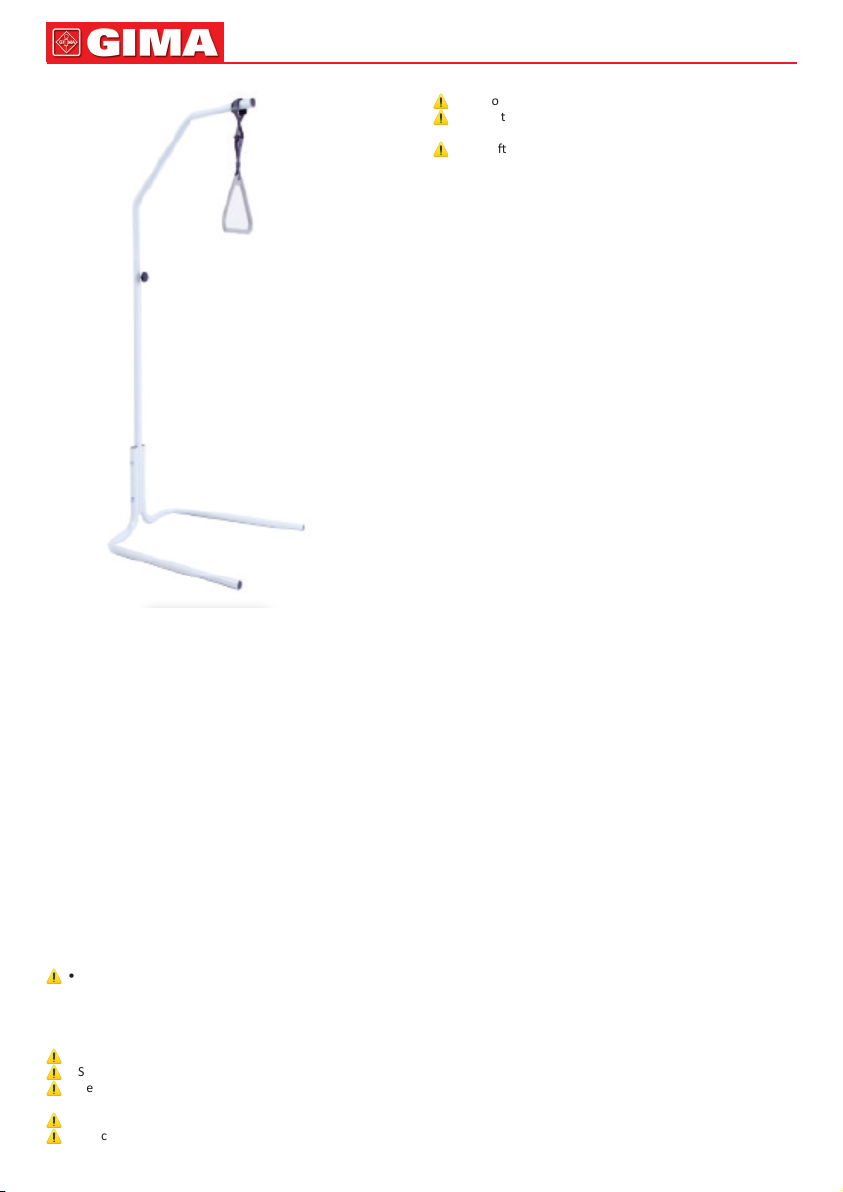
Support device with ground base, adaptable to any bed. Struc-
ture in tubular steel painted that can be posioned according to
the paent’s needs and triangle with strap to adjust its height.
CAUTION (!):
• In case of damage or malfuncon of your product DOT NOT
use the product and please contact the authorized and trained
representave.
• Do not use the product for a purpose not indicated in this manual
• LONGWAY Medical declines all responsibilies for any conse-
quences resulng from an incorrect use of this product and from
unauthorized alteraon to the frame of the product.
• LONGWAY Medical reserves the right to change the informa-
on contained in this document without previous noce
• There is a ± 3%. Deviaon for the Dimensions of the Products.
GENERAL SAFETY WARNINGS:
⚠
• If you don’t read this user manual, it is preferable not to
use this product or another available part. If you don’t un-
derstand the usage or the precauons, please contact with
the dealer or the proper technical person before you use the
product because may be caused damage.
⚠
• Keep away the product from heat sources.
⚠
• Save this document for future reference.
⚠
• Be careful when children are nearby and do not allow the
children to play with the product.
⚠
• Do not exceed the maximum payload.
⚠
• Special care is always required where there are moving
parts that can cause limb entrapment and injury.
⚠
• Do not aempt to li the product by any removable parts.
⚠
• Do not use the product seat for a dierent purpose from
the intended one.
⚠
• The liing poles are not intended to carry the full weight
of an individual.
DECLARATION OF CONFORMITY:
We are solely responsible for declaring that the Medical Devic-
es menoned in this statement are of Low-Risk Class (Class I)
and comply with the requirements of the European Regulaon
745/2017 and where appropriate, the standards and legislaon
referred to.
BEFORE USE:
• Check if the frame is damaged in order to guarantee a safe use
of the product. (There are no cracks or fractures in the frame).
• In case of damage do not use the product and contact your
dealer for further instrucons.
• Check, if the product is properly assembled and all screws are
secure and well screwed on.
• Always check the condion of wear for the mechanical parts
to ensure the absolute safe use of the product for people and
objects
ENVIRONMENTAL PROTECTION:
If one day you nd that your product needs to be replaced or it is
no longer working for you, consider protecng the environment:
1) Do not dispose your product along with the rest of the public
waste (this is also the meaning of the shown recycling sign).
2) Contact your Public Authories and they will instruct you of
the Recycling centers to which your product must be disposed.
3) Correct disposal of your product helps the protecon of the
environment as well as the recycling of the product’s compo-
nents.
MAINTENANCE, CLEANING & DISINFECTION
INFORMATION:
To guarantee a safe use and adequate standard of hygiene, the
user should perform these procedures before every use.
The user has to make certain of the structural integrity of the de-
vice and its components. For cleaning and sanitaon procedures,
follow the steps below:
1. Gloves should be worn.
2. Remove the evident dirt before to proceed with the most ac-
curate disinfecon.
3. Use water or mild detergent for the cleaning procedure.
4. Wipe with a dry and clean cloth.
5. DO NOT leave the product wet.
6. Do not use chemical cleaners to clean the frame. This could
cause damage to the surface of the product
7. If you need to disinfect the product, use a common, mild dis-
infectant
8. Protect the item from scratches, cuts and punctures.
9. Lubricaon: the product has designed for a minimal mainte-
nance; however, check every six months the lubricaon on the
moving & mechanical parts, this would ensure an opmal du-
raon.
ASSEMBLING:
NOTE: The illustraons for the assembly are indicave to make
the assembly easier. Μay will be dierences from reality.
The support with LW10207 base is delivered disassembled and
requires the following assembly operaons:
1. Use 2*M10*115mm screws to x the straight tube between
the le and right leg tubes;