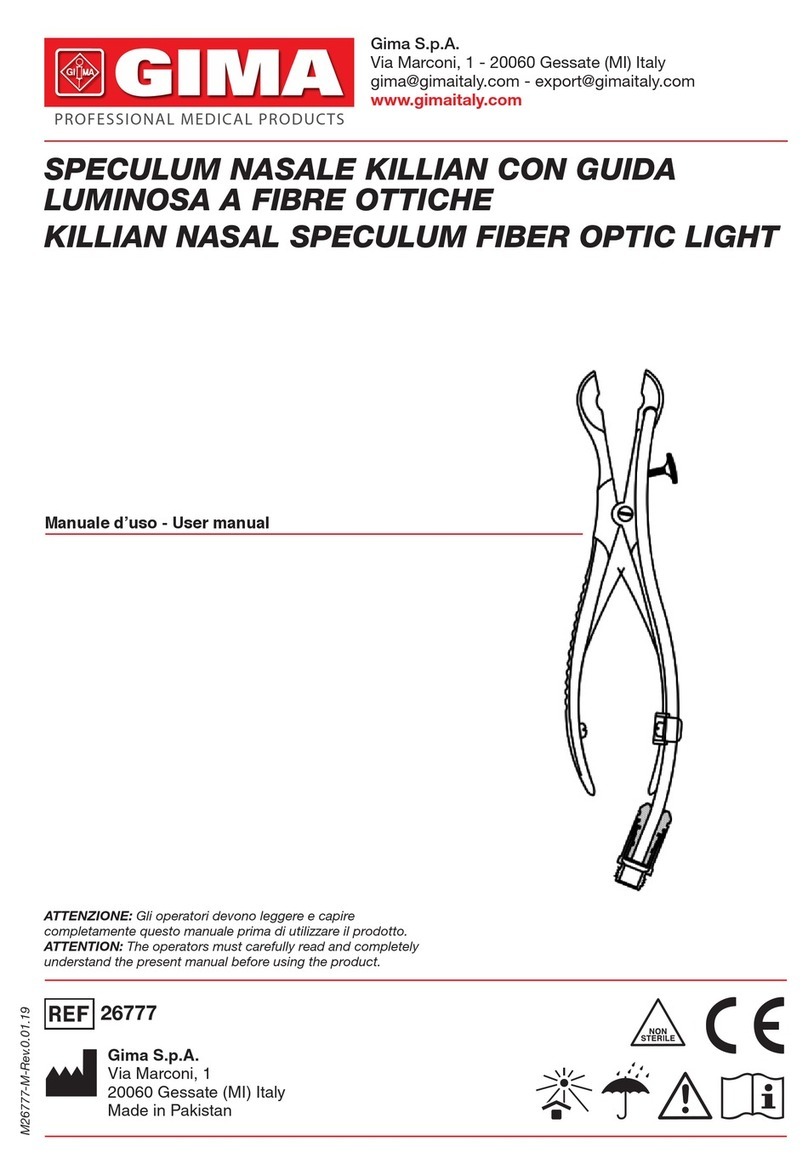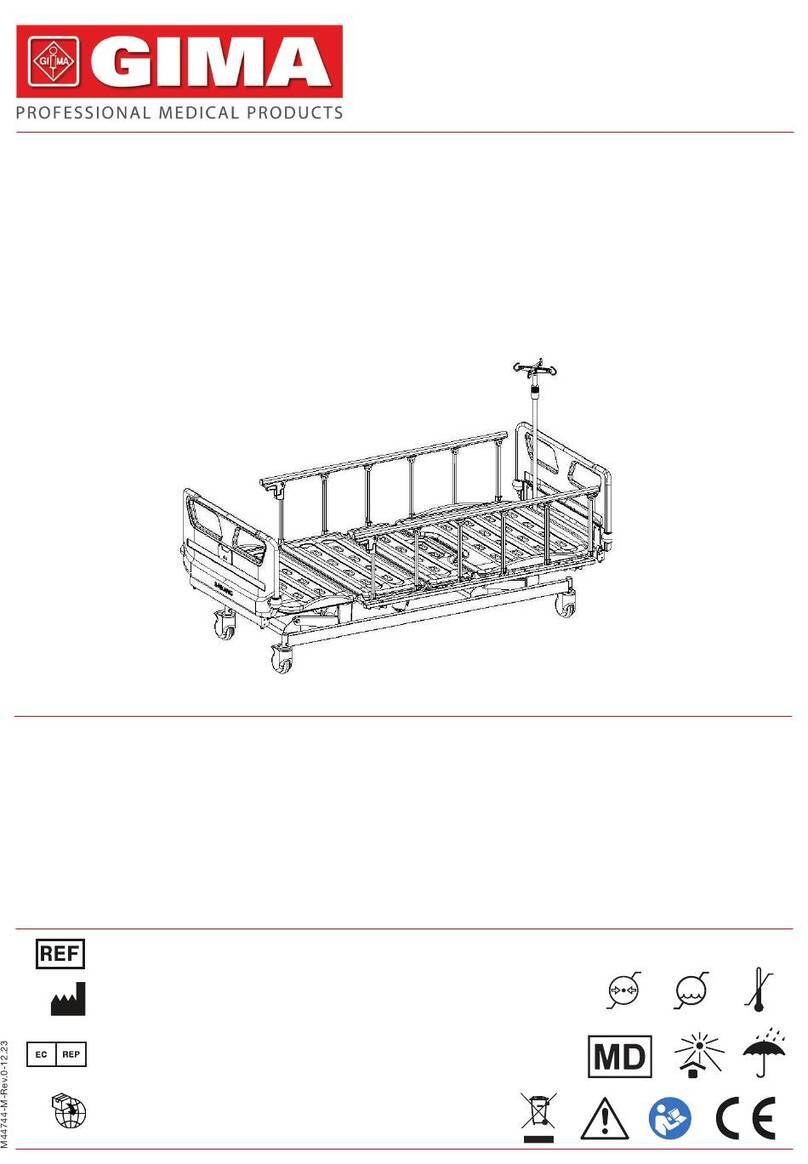
15 ENGLISH
SUPERVEGA EVO is a desk-type electric suction unit for the aspiration of body liquids (such as mucus ,
phlegm and blood), oral, nasal and tracheal aspiration in adults or children. Its shape, elegantly narrow, is
motly designed for tting into ambulance care and emergency use.
This device has been designed to offer ease of transport and continuous use, thanks to an electronic system
that manages the power supply. The large LCD display facilitates the use of the device and increases suction
by adjusting the control buttons.
The signal on the LCD screen, placed on the front panel, allows you to monitor the charge status of the
Lithium battery. The lithium battery and the innovative Feedback system, which guarantees a smart use by
automatically controlling and adjusting the suction power, allow the battery to increase its autonomy and
decrease the noise produced.
The “PROXIMITY” function allows the device to be activated/deactivated via an infra-red proximity sensor
(detecting the presence of the hand from a distance of tenths of centimetres without touching the suction
unit) and it prevents and avoids possible crosscontamination between patients as they are treated in turns.
Made of highly heat resistant, electrically insulated plastic material in conformity with the latest European
safety standard, the product is supplied with a complete polycarbonate autoclavable jar with overow valve.
GENERAL WARNING
Read instruction manual carefully before use.
A careful and correct use ensures optimal operation of the device.
The device is for use by qualied personnel (surgeon / professional nurse / assistant). The use of the device
at home is restricted to an adult in full possession of mental faculties and / or home carers.
The instrument must not disassembled. For technical service always contact Gima S.p.A.
BASIC SAFETY RULES
1. When opening the packaging, check the integrity of the device, paying particular attention should there
be any damage to the plastic parts, which could make accessible the internal parts of the device under
voltage, and to breakage and/or peeling of the power cable. In case of any damage, do not connect the
plug to the electrical socket. To replace it, contact Gima technical service.
2. Before connecting the device, always check that the electrical details indicated on the data label and the
type of plug used correspond to those of the electrical network to which you intend to connect it.
3. Comply with the safety rules indicated for electrical equipment and in particular:
- Use only original accessories and components, supplied by the manufacturer Gima in order to ensure
maximum efciency and safety of the device.
- Always use the medical device with the antibacterial lter supplied by the manufacturer Gima in order to
ensure maximum efciency and safety of the device.
- Never immerse the device in water or other liquids.
- Do not use the device in environments where there are ammable anaesthetic mixtures with air, oxygen
or nitrous oxide which could cause explosions and/or res.
- Do not place the aspirator on unstable operating surfaces whose accidental fall could cause malfunctions
and/or breakages. Should there be any damage to the plastic parts, which could make accessible the
internal parts of the device under voltage, do not connect the plug to the electrical socket. Do not
try to operate the device before it has undergone a thorough check by qualied personnel and/or by the
Gima technical service.
- Do not use the device in environments where there are ammable anaesthetic mixtures with air, oxygen
or nitrous oxide which could cause explosions and/or res.
- Avoid touching the device with wet hands and in any case always ensure that the device does not come
into contact with liquids.
- Prevent children and/or incapacitated users from using the device without due supervision.
- Don’t pull the power supply cable to disconnect the plug remove the plug from the mains socket correctly.
- Store and use the device in weatherproof environments and away from any heat sources.
- The device cannot be used to drain chest uids.
- In general, it is inadvisable to use single or multiple adapters and/or extensions. Should their use be
necessary, you must use ones that are in compliance with safety regulations, however, taking care not to
exceed the maximum power supply tolerated, which is indicated on the adapters and extensions.
- Never leave the appliance near water, do not immerse it in any liquid. If the device has fallen into water,
unplug it before you hold it. Do not use the appliance if the plug or AC / DC power supply is damaged or