23
2.2 Major Applications and Scope
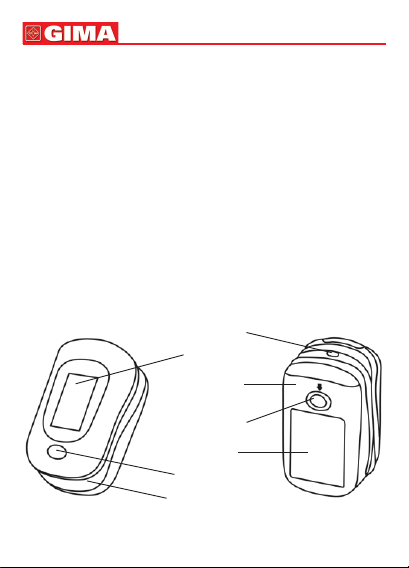
The Fingertip Oximeter is compact, convenient to use and carry and
with low power consumption. You just need to put the ngertip into the
sensor of the device, the SpO2value will appear on the screen imme-
diately.
The Fingertip Oximeter can detect SpO2and pulse rate through pa-
tient’s nger.
This device is applicable to home, hospital (including internal medicine,
surgery, anesthesia, pediatrics, emergency room etc.), oxygen bar, the
community medical center, alpine area and it also can be used before or
after sports, and the like.
This device is not appropriate to be used for continuous mon-
itoring.
2.3 Principle of Measurement
Based on Lamber-Beer law, the light absorbance of a given substance is
directly proportional with its density or concentration. When the light
with certain wavelength emits on human tissue, the measured intensity
of light after absorption, reecting and attenuation in tissue can reect
the structure character of the tissue by which the light passes. Due to
that oxygenated hemoglobin (HbO2) and deoxygenated hemoglobin
(Hb) have different absorption character in the spectrum range from red
to infrared light (600nm~1000nm wavelength), by using these character-
istics, SpO2can be determined. SpO2measured by this Pulse Oximeter
is the functional oxygen saturation -- a percentage of the hemoglobin
that can transport oxygen. In contrast, hemoximeters report fractional
oxygen saturation – a percentage of all measured hemoglobin, including
dysfunctional hemoglobin, such as carboxyhemoglobin or metahemo-
globin.
Clinical application of pulse oximeters: SpO2is an important phys-
iological parameter to reect the respiration and ventilation function,
so SpO2monitoring used in treatment has become more popular. (For
example, such as monitoring patients with serious respiratory disease,
patients under anesthesia during operation and premature and neo-
natal infants) The status of SpO2can be determined in timely manner
ENGLISH