GB
11
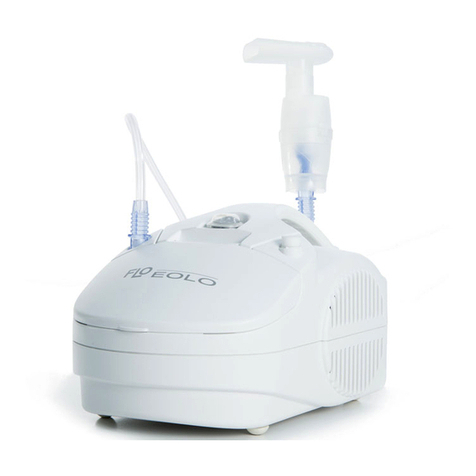
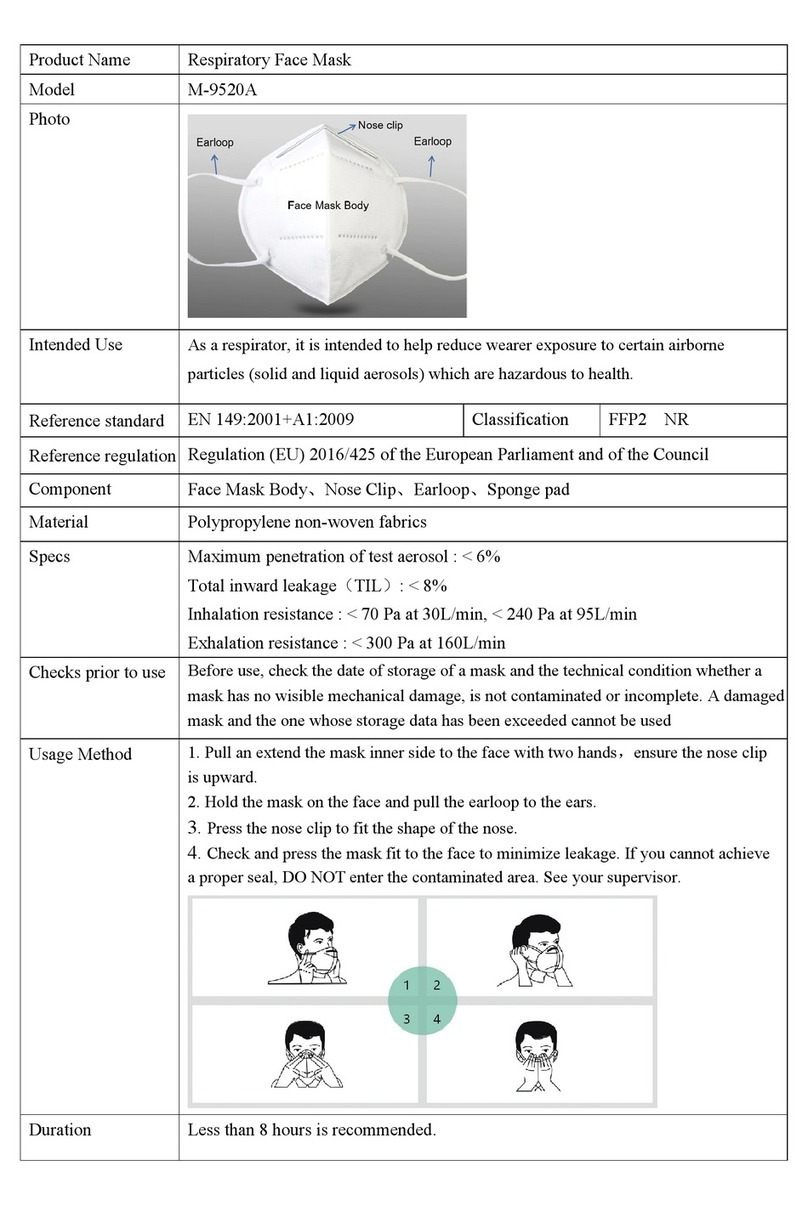
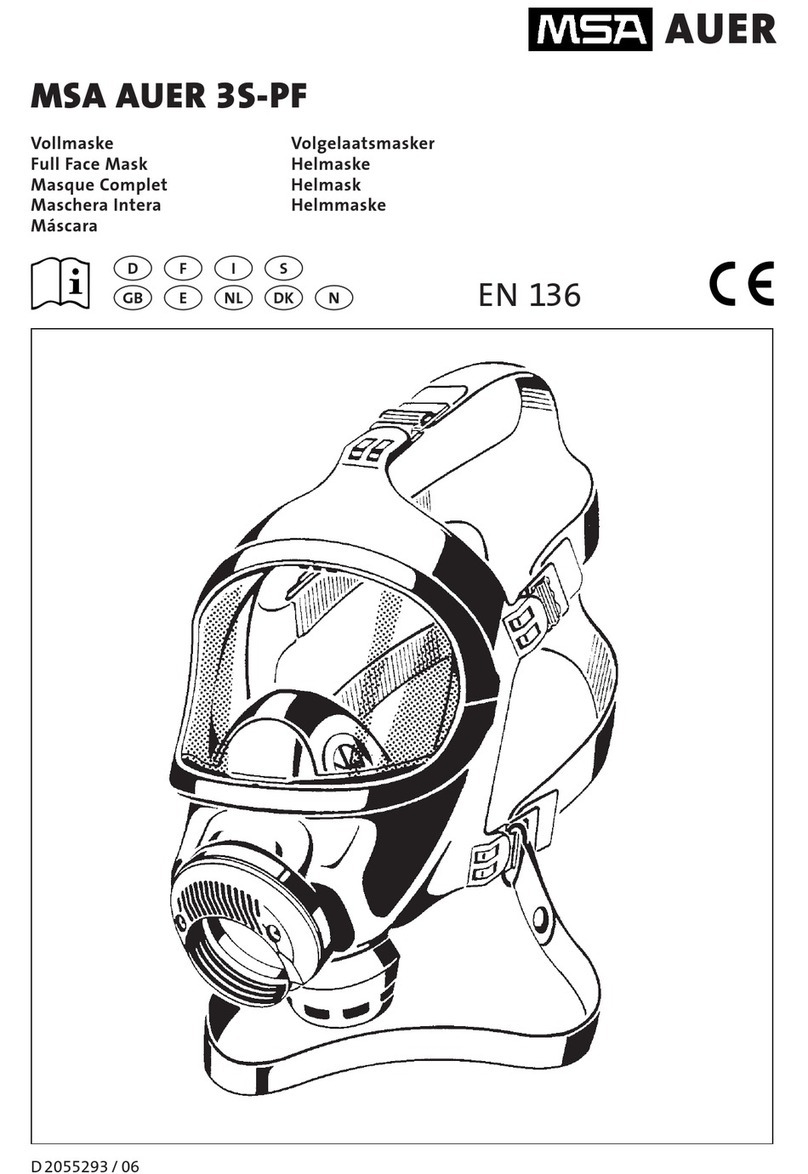
EOLO is an electrical fed compressor with aerosol therapy atomiser.
The instrument is designed for easy transport and handing and is recommended for atomising antibiotics and bronchodilator
drugs. The high thermal insulated plastic body complies with European Safety Rules. The medical device is designed for continuous
use
READ INSTRUCTION MANUAL CAREFULLY BEFORE USE
DRUG ADMINISTRATION MUST BE UNDER MEDICAL CONTROL
THE INSTRUMENT MUST NOT BE DISASSEMBLED. FOR A TECHNICAL SERVICE ALWAYS CONTACT GIMA
1. On opening the packaging, check the integrity of the appliance, paying particular attention to the presence of damage to the
plastic parts, which may make access possible to internal live parts and also to breakage and / or peeling of the power supply
cable. In these cases don’t connect the plug to the electric socket. Carry out these controls before each use;
2. before connecting the appliance always check that the electric data indicated on the data label and the type of plug used,
correspond to those of the mains electricity to witch it’s to be connected;
3. Never leave the appliance inserted if not necessary disconnect the plug from the mains power supply when it is not being
used;
4. Respect the safety regulations indicated for electrical appliances and particularly:
Only use original accessories and components provided by the manufacturer GIMA to guarantee the highest efficiency and
safety of the device;
Never immerge the appliance into water;
Position the appliance on flat stable surfaces;
Position the device in a way that the air inlets on the back aren’t obstructed;
Never use the device in environments which have anaesthetic mixtures inflammable with air, oxygen or nitric oxide;
Don’t touch the device with wet hands and always prevent the appliance coming into contact with liquids;
The use of this device by children and / or incompetent person always requires the careful surveillance of an adult in
possession of their full mental faculties;
The medical device, and most of all the nebulae, must be kept out of children’s reach as it contains small parts hat could be
swallowed;
Don’t leave the appliance connected to the power supply socket when not in use;
Don’t pull the power supply cable to disconnect the plug remove the plug from the mains socket correctly;
Store and use the device in places protected against the weather and far from any sources of heat. After each use, it is
recommended to store the device in its own box away from dust and sunlight.
In general, it is inadvisable to use single or multiple adapters and/or extensions. Should their use be necessary, you must
use ones that are in compliance with safety regulations, however, taking care not to exceed the maximum power supply
tolerated, which is indicated on the adapters and extensions.
5. For repairs, exclusively contact GIMA technical service and request the use of original spare parts.
Failure to comply with the above can jeopardise the safety of the device;
6. This medical device must be destined exclusively for the use for witch it has been designed ad described in this
manual. It must therefore be used as an aerosol therapy system. Any different use must be considered incorrect and
therefore dangerous; the manufacturer cannot be considered liable for damage caused by improper, incorrect and / or
unreasonable use or if the appliance is used in electrical plants that are not in compliance with the regulations in force;
7. The medical device requires special precautions regarding electromagnetic compatibility and must be installed and used in
accordance with the information provided with the accompanying documents: the Eolo device must be installed and used
away from mobile and portable RF communication devices (mobile phones, transceivers, etc.) that may interference with the
said device.
8. Store the accessories out of reach of children. Children and people with learning difficulties must only use the medical device
under the strict supervision of an adult with full mental faculties. Keep the ampoule out of reach of children under 36 months
as it contains small parts that may be swallowed accidentally. Never leave the device unattended in places accessible to
minors and / or the disabled.
9. WARNINIG: None of electric or mechanical parts have been designed to be repaired by customers or end-users. Don’t open
the device, do not mishandle the electric / mechanical parts. Always contact GIMA technical assistance.
10. Do not leave the device unattended in places accessible to children and / or persons not in full possession of their mental
faculties as there is a risk of strangulation with the air tube;
11. The medical device may come into contatct with the patient via the nubuliser / masks / mouthpiece and / or nosepiece,
components compliant with the requirements of regulation ISO 10993-1: therefore, no allergic reaction and skin irritation
may occur.
12. The product and its parts are biocompatible in accordance with the requirements of regulation EN 60601-1.
13. Operation of the device is very simple and therefore no further explanations are required other than those indicated in the
following user manual.