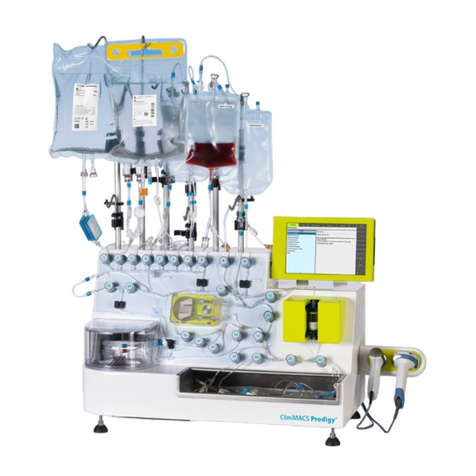
Miltenyi Biotec CliniMACS Prodigy User manual

CliniMACS Prodigy®
User Manual
CliniMACS Prodigy® | User Manual

The CliniMACS System components, including Reagents, Tubing Sets, Instruments, and PBS/EDTA Buer, are designed,
manufactured and tested under a quality system certied to ISO 13485. In the EU, the CliniMACS System components are
available as CE-marked medical devices for their respective intended use, unless otherwise stated. In the US, the
CliniMACS CD34 Reagent System, including the CliniMACS Plus Instrument, CliniMACS CD34 Reagent, CliniMACS Tubing
Set TS and CliniMACS Tubing Set LS, and the CliniMACS PBS/EDTA Buer, is FDA approved as a Humanitarian Use Device
(HUD), authorized by U.S. Federal law for use in the treatment of patients with acute myeloid leukemia (AML) in rst
complete remission. The eectiveness of the device for this indication has not been demonstrated. All other products of
the CliniMACS Product Line are available for use only under an approved Investigational New Drug (IND) application or
Investigational Device Exemption (IDE). In Australia, the following components of the CliniMACS Prodigy System are
included in the Australian Register of Therapeutic Goods (ARTG) and are therefore approved for supply: CliniMACS
Prodigy, CliniMACS CD34 Reagent, CliniMACS Prodigy Tubing Sets, and CliniMACS PBS/EDTA Buer. Only those products
which are included in the ARTG may be used in Australia. CliniMACS MicroBeads are for research use only and not for
human therapeutic or diagnostic use.
Unless otherwise specically indicated, Miltenyi Biotec products and services are for research use only and not for
therapeutic or diagnostic use.
Copyright © 2021 Miltenyi Biotec and/or its aliates. All rights reserved.
No part of this publication may be reproduced, stored in a retrieval system, transmitted, published, or distributed in any form
or by any means, electronically, mechanically, by photocopying, microlming, recording, or otherwise, without the prior
written consent of Miltenyi Biotec; however, notwithstanding the foregoing, the owners of the CliniMACS Prodigy System
may make copies solely for purposes of training personnel in the use and servicing of the unit within their business or
organization.
CentriCult, CliniMACS, CliniMACS Prodigy, MACS, the Miltenyi Biotec logo, PepTivator, and TexMACS are registered trademarks
or trademarks of Miltenyi Biotec B.V. & Co.KG and/or its aliates in various countries worldwide. All other trademarks
mentioned in this document are the property of their respective owners and are used for identication purposes only.

CliniMACS Prodigy®
User Manual
Issued: 2021-03
38001/05
Miltenyi Biotec B.V. & Co. KG
Friedrich-Ebert-Straße 68
51429 Bergisch Gladbach
Germany
Miltenyi Biotec Technical Support:
+49 2204 8306-3803
technicalsuppor[email protected]
www.miltenyibiotec.com

2

3
Essential information
This user manual provides instructions, warnings, precautions, and other
important information for the use of the CliniMACS Prodigy® as well as warnings
and precautions concerning the handling of biohazardous materials and cellular
starting product. For details on processes run on the instrument, refer to the
CliniMACS Prodigy User Manual for the respective application.
The operation of the CliniMACS Prodigy System must be performed by
trained operators only. Before putting the system into operation, care-
fully read and understand the safety information, warnings, precautions,
and instructions for proper operation of the CliniMACS Prodigy provided
in the instructions for use of the CliniMACS Prodigy System components
(including, without limitation, the safety information in this user manual,
chapter3 “Important safety information”) and in any safety-related
recommendations issued by Miltenyi Biotec. Pay special attention to all
warnings displayed on the instrument or provided with consumables and
accessories. The operator must adhere to all instructions and procedures
at all times during the operation of the instrument, conrming that all
safety information, warnings, precautions, and instructions are
observed. Failure to follow the safety information, warnings,
precautions, and instructions contained in the instructions for use could
result in instrument malfunction, property damage, personal injury, and/
or death. Equipment safety may be compromised if the instrument is not
used according to the manufacturer’s instruction.
Retain the instructions for use for future reference. They should be kept
accessible and readily available, together with all other safety and
operating documentation, during the entire life cycle of the instrument
for all personnel responsible for installation, operation, and
maintenance.

4

5
Table of contents
1 Introduction 9
1.1 General information 9
1.2 Service information 10
1.2.1 CliniMACS Prodigy® Information 10
1.2.2 Technical support 10
2 Glossary 11
2.1 Graphical depiction 11
2.2 Glossary of symbols 12
2.2.1 Safety symbols 12
2.2.2 Symbols used for labeling of products 13
2.2.3 Glossary of terms 16
3 Important safety information 27
3.1 Safety instructions for the CliniMACS Prodigy® 30
3.1.1 Usage and installation 30
3.1.2 Electric hazards 31
3.1.3 Strong magnetic eld 33
3.1.4 Optical radiation hazards 33
3.1.5 Chemical and biological hazards 34
3.1.6 Mechanical hazards 34
3.1.7 Gas hazards 35
3.1.8 Hot surfaces 35
3.1.9 Servicing and transport 35
3.2 Position of safety symbols 37

6
4 The CliniMACS Prodigy® 39
4.1 Regulatory information 39
4.1.1 The CliniMACS Prodigy® within the CliniMACS Prodigy
Cell Separation System 40
4.1.2 The CliniMACS Prodigy® within the CliniMACS Prodigy
Cell & Gene Therapy Manufacturing System 41
4.2 Technical data 42
4.3 Components of the CliniMACS Prodigy® 44
4.3.1 Housing and bag hangers 47
4.3.2 Monitor 48
4.3.3 Peristaltic pump 48
4.3.4 Magnet unit 49
4.3.5 CentriCult™ Unit 49
4.3.6 Gas mix unit 51
4.3.7 Sensors 52
4.3.8 Pinch valves 52
4.3.9 Connector panel 53
4.4 Accessories for the CliniMACS Prodigy® 55
4.4.1 MACS® TubeSealer 55
4.4.2 Bar code reader 55
4.4.3 CliniMACS Prodigy® Supplementary Bag 56
4.4.4 CliniMACS® Electroporator 56
4.4.5 CliniMACS® Formulation Unit 57
Table of contents (continued)

7
4.5 Unpacking and installation 57
4.5.1 Scope of supply 57
4.5.2 Transport 58
4.5.3 Positioning 58
4.6 Cleaning and disinfection 59
4.7 Maintenance 60
4.8 Disposal 60
5 The CliniMACS Prodigy® Software 61
5.1 Touchscreen 61
5.1.1 Use of touchscreen 61
5.1.2 General setup menu 62
5.2 Login, logout, and emergency stop 62
5.2.1 Login 63
5.2.2 Logout 63
5.2.3 Emergency Stop 64
5.3 The graphic user interface 64
5.3.1 Operating controls 64
5.3.2 Menus 70
5.4 Application Services 78
5.4.1 User Management 79
5.5 Alarm management 86
Table of contents (continued)

8
6 The CliniMACS Prodigy® System 89
6.1 The CliniMACS Prodigy® System components 89
6.2 Additional materials and equipment 90
6.3 Limitation 90
6.4 Warnings and precautions regarding the process 91
6.5 Warnings and precautions regarding the handling of
biohazardousmaterial 92
6.6 Warnings and precautions regarding the cellular starting product 93
7 Troubleshooting 95
7.1 Instrument malfunction or process failure 95
7.2 Instrument cleaning after leakage 95
8 Legal notes 97
8.1 Limited warranty 97
8.2 Trademarks 98
Appendix 99
Guidanceandmanufacturer’sdeclaration
onelectromagnetic compatibility
Table of contents (continued)

9
1
INTRODUCTION
1
1
Introduction
1.1 General information
The CliniMACS Prodigy System oers a set of tools that enables high-quality and
standardized cell processing for many dierent applications. The system is
based on the magnetic cell separation technology (MACS® Technology)
developed by Miltenyi Biotec.
The CliniMACS Prodigy represents the next generation in automated cell and
liquid processing. With its integrated uid transfer, centrifugation, and magnetic
separation capability, the instrument enables fully automated cell processing
and culture applications, as well as uid handling. The instrument oers
advanced integrated solutions to streamline cell-processing workows. It
provides a exible platform enabling the separation of virtually any cell type, as
well as customized separation protocols that meet specic process require-
ments. The dierent applications run on the instrument require the use of
specic CliniMACS Prodigy System components as well as additional materials
and equipment, as described in the CliniMACS Prodigy User Manual issued for
the respective application (see also section 6.1).

10
1.2 Service information
1.2.1 CliniMACS Prodigy® Information
Record the model and serial number located on the back of the CliniMACS
Prodigy® below. Refer to these numbers whenever requesting information
about the instrument or when requesting instrument service.
Reference no. (REF):
Serial no. (SN):
The software version is shown during the start-up phase of the instrument.
1.2.2 Technical support
For information or support, contact Miltenyi Biotec Technical Support:
Miltenyi Biotec B.V. & Co. KG
Friedrich-Ebert-Straße 68
51429 Bergisch Gladbach
Germany
+49 2204 8306-3803
technicalsuppor[email protected]
Visit www.miltenyibiotec.com for local Miltenyi Biotec Technical Support
contact information.

11
GLOSSARY
2
2
Glossary
2.1 Graphical depiction
The following chart depicts the panels used in this user manual to inform the
user about potential risks if the outlined warnings and precautions are not
followed. The hazard level classies the hazard, as described below. The level,
type, and source of the hazard, as well as potential consequences, prohibitions,
and measures are indicated as follows. Icons on the left side specify the risk.
Indicates a hazardous situation which, if not avoided, could result in
death or serious injury.
Indicates a hazardous situation which, if not avoided, could result in minor or
moderate injury.
Addresses practices or information not related to personal injury but may lead
to property damage.
IMPORTANT
Advises the user of important practices or information not related to personal injury
nor property damage.

12
2.2 Glossary of symbols
2.2.1 Safety symbols
General warning sign
Warning: Electricity
Warning: Magnetic eld
Warning: Optical radiation
Warning: Biological hazard
Warning: High sound pressure level
Warning: Crushing of hands
Warning: Hot surface

13
GLOSSARY
2
2.2.2 Symbols used for labeling of products
Medical Device
European conformity approval with ID number 0123 (ID
number of Notied Body: “TÜV SÜD Product Service GmbH,
Munich”).
Eurasian Conformity mark
NRTL certication mark: Product meets consensus-based
standards of safety, required by the Occupational Safety/
Health Administration (OSHA), determined by the Nationally
Recognized Testing Laboratories (NRTL) TÜVSÜD
Consult instructions for use
Caution
Manufacturer
Date of manufacture
Protective conductor terminal (ground)
Non-ionizing radiation
Separate collection for waste of electrical and electronic
equipment
Fuse

14
Keep dry
Fragile, handle with care
This way up
Power OFF
Power ON
Installation date
Do not re-use.
Do not use if package is damaged
The packaging is PVC free
Use-by date
Temperature limit
Non-pyrogenic uid path
Batch code
Part number
Contents of the packaging

15
GLOSSARY
2
Catalogue number
Serial number
Unique Device Identication
Sterilized using aseptic processing techniques
Sterilized using steam or dry heat
Sterilized using irradiation
Non-sterile
Single sterile barrier system
Single sterile barrier system with protective packaging outside
Phone
Fax
E-Mail
Website

16
2.2.3 Glossary of terms
Alarm connector Relay circuit connector for the connection of the
instrument to an external alarm system
Antigen Bag Bag containing antigen, part of the CliniMACS
ProdigyTS 500
Apheresis A method of collecting blood in which the whole
blood is withdrawn, a desired component selected
and retained, and the remainder of the blood
returned to the donor
Application Bag Bag for cellular starting product, part of CliniMACS
Prodigy Tubing Sets
Connector for compatible external devices, currently
non-functional
AUX 5 pin connector See TubeSealer port
Bag compartment Compartment of the CliniMACS Prodigy for the
storage of bags during instrument operation
Bag hanger Component of the CliniMACS Prodigy on which
elements of the CliniMACS Prodigy Tubing Sets, such
as buer bags, application bags, or vials are
mounted
Bar code Machine-readable representation of data, e.g., part
number, batch code, or use-by date, indicated on
required materials
Bar code reader The bar code reader serves as a data input device to
scan bar codes printed on, e.g., CliniMACS Materials,
needed for process execution with the CliniMACS
Prodigy System.
Bone marrow aspirate Sample of bone marrow taken by insertion of a
needle through the bone drawn into a syringe
CAN Controller Area Network
CAN connector Connector used for service purposes only
AUX 3 pin connector
(for CliniMACS Prodigy
manufactured until July 2020)

17
GLOSSARY
2
Cell-containing product used as starting material for
the CliniMACS Prodigy Applications, e.g.,
leukapheresis harvest, PBMCs, or bone marrow
CentriCult™ Unit The CentriCult Unit, as a part of the CliniMACS
Prodigy, comprises a cell processing compartment
featuring the following components: Layer
Detection Camera, microscope camera, heat
exchange unit, chamber lock adapter, and the lid of
the unit. This compartment can be temperature and
atmosphere controlled for centrifugation,
incubation, and cell cultivation.
CentriCult Unit lid Protection cover locking the CentriCult Unit
Centrifugation sensor Part of the CentriCult Unit to control the
centrifugation speed
Chamber Compartment for fractionation, washing, and
labeling by centrifugation and for cultivation of cells,
part of the CliniMACS Prodigy Tubing Sets
Chamber drive unit Unit which drives the chamber within the CentriCult
Unit
The chamber lock adapter holds the chamber of the
tubing set and connects it to the chamber drive unit
Channel clip Blue clip holding the tubing outside the CentriCult
Unit in position
Check valve Check valves are two-port valves, meaning they
have two openings in the body, one for uid to enter
and the other for uid to leave. An important
concept in check valves is the cracking pressure
which is the minimum upstream pressure at which
the valve will operate.
Cleaning solution Aqua bidest for injection use. The cleaning solution
is used for cleaning the inner tubing and chamber of
the tubing set during specic cleaning process steps.
The cleaning solution is not part of the CliniMACS
Prodigy System.
Cellular starting
product
Chamber lock adapter

18
Cleaning solution bag Bag containing the cleaning solution. The cleaning
solution bag is not part of the CliniMACS Prodigy
TubingSets.
Buer used for cell preparation and cell separation
with the CliniMACS System: PBS (phosphate-
buered saline), supplemented with 1 mM EDTA, pH
7.2. Before use, CliniMACS PBS/EDTA Buer must be
supplemented with pharmaceutical-grade HSA to a
nal concentration of 0.5% (weight/volume, i.e. 5 g
HSA per liter buer). HSA-supplemented buer is
called process buer.
The CliniMACS Electroporator is a fully automatic
instrument for the electroporation of eukaryotic
cells in combination with the CliniMACS Prodigy.
After electroporation, cells are further processed by
the CliniMACS Prodigy.
Buer used for the electroporation of cells with the
CliniMACS Electroporator
The CliniMACS Formulation Unit is an instrument
accessory for the automated nal formulation and
sampling of eukaryotic cells in combination with the
CliniMACS Prodigy. The CliniMACS Formulation Unit
enables the user to take cell samples during or after
processing by the CliniMACS Prodigy.
The CliniMACS Prodigy is an instrument for
automated cell processing. This instrument oers
integrated solutions for cell processing workows:
from cell separation through cell culture to
formulation of the nal product.
The CliniMACS Prodigy Supplementary Bag is
intended to help reduce the user's contact to
accidently emitted, potentially infectious sample
material out of the CliniMACS Prodigy Tubing Set.
CliniMACS PBS/EDTA
Buer
CliniMACS
Electroporator
CliniMACS
Electroporation Buffer
CliniMACS
Formulation Unit
CliniMACS Prodigy
CliniMACS Prodigy
Supplementary Bag
Table of contents
Other Miltenyi Biotec Laboratory Equipment manuals

Miltenyi Biotec
Miltenyi Biotec CliniMACS Plus System User manual

Miltenyi Biotec
Miltenyi Biotec MACS MiniSampler S User manual

Miltenyi Biotec
Miltenyi Biotec MACSQuant Buffer Supply Station User manual

Miltenyi Biotec
Miltenyi Biotec CliniMACS Plus System User manual

Miltenyi Biotec
Miltenyi Biotec MACSQuant Tyto User manual

Miltenyi Biotec
Miltenyi Biotec MACSima User manual

Miltenyi Biotec
Miltenyi Biotec MultiMACS Cell24 Separator Plus User manual
Miltenyi Biotec
Miltenyi Biotec autoMACS Pro Separator Manual

Miltenyi Biotec
Miltenyi Biotec MACSQuant Tyto Sorter User manual

Miltenyi Biotec
Miltenyi Biotec MACSmix Tube Rotator User manual