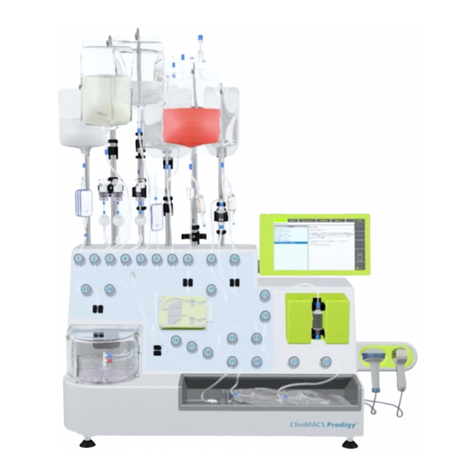
Miltenyi Biotec CliniMACS Formulation Unit User manual




















This manual suits for next models
1
Table of contents
Other Miltenyi Biotec Laboratory Equipment manuals
Miltenyi Biotec
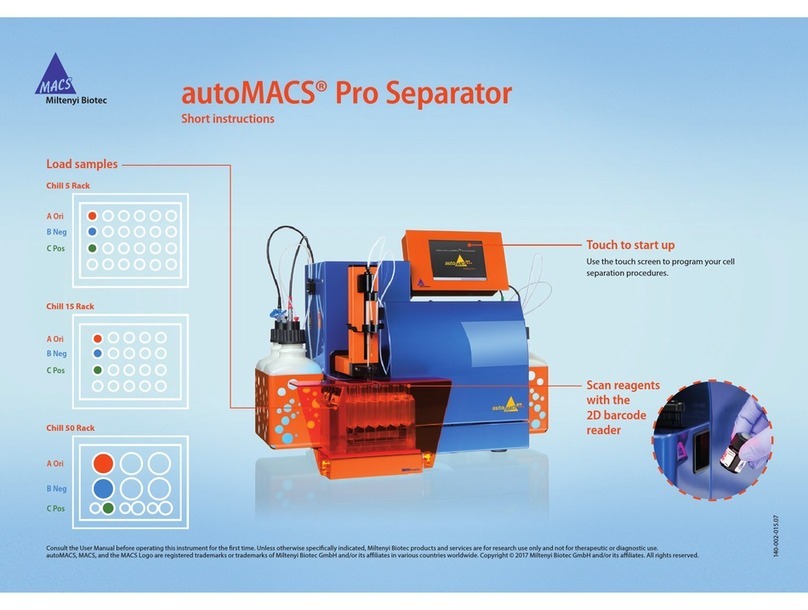
Miltenyi Biotec autoMACS Pro Separator Manual

Miltenyi Biotec
Miltenyi Biotec MACSQuant X User manual

Miltenyi Biotec
Miltenyi Biotec MACSQuant Buffer Supply Station User manual

Miltenyi Biotec
Miltenyi Biotec CliniMACS Plus System User manual

Miltenyi Biotec
Miltenyi Biotec MACSQuant Tyto User manual

Miltenyi Biotec
Miltenyi Biotec MACSQuant Tyto Sorter User manual

Miltenyi Biotec
Miltenyi Biotec MACSima User manual

Miltenyi Biotec
Miltenyi Biotec MACS MiniSampler S User manual

Miltenyi Biotec
Miltenyi Biotec MultiMACS Cell24 Separator Plus User manual

Miltenyi Biotec
Miltenyi Biotec CliniMACS Plus System User manual
Popular Laboratory Equipment manuals by other brands

NuAire
NuAire LabGard NU-540-300E Operation and maintenance manual

Thermo Scientific
Thermo Scientific thermoscientific Vanquish Pumps C operating manual

Selecta
Selecta BLOOD-BANK A instruction manual

ESCO Technologies
ESCO Technologies ETS-LINDGREN SuiteSentry Operation and installation manual

Thermo Savant
Thermo Savant SPEEDVAC SPD Series instruction manual

Molecular Devices
Molecular Devices StakMax user guide