Mainstream EtCO2 Setup
9650-1212-01 Rev. E 5
2. Press the sensor and airway adapter together until
they click.
3. Turn the Selector switch on the E Series unit to
MONITOR (ON for AED units).
4. Wait for the airway adapter and sensor to warm up.
The unit will display a WARM UP message for
approximately one minute while the sensor and
adapter warm to operating temperature. The
message disappears when the sensor is ready to
use.
Note Warm up time varies with ambient temperature of
the sensor.
5. If the unit displays the CHECK CO2 ADAPTER
message, follow steps a through c.
a. Verify proper connection of the adapter to the
sensor.
b.Verify that the airway adapter windows are clean
and dry.
c. If the adapter is properly connected, and the
windows are clean and dry, then zero the adapter
as described in the next section, “Zeroing the
Mainstream CAPNOSTAT 5 CO2Sensor/Airway
Adapter.”
Zeroing the Mainstream CAPNOSTAT 5 CO2
Sensor/Airway Adapter
Adapter zeroing compensates for the optical differences
between airway adapters and should be performed after
switching between single patient use and reusable
airway adapters, in order to obtain accurate readings.
Zeroing is also recommended the first time a particular
CAPNOSTAT 5 CO2 sensor is connected to the unit.
1. Place the sensor with the adapter installed away from
all sources of CO2 (including the patient’s – and your
own – exhaled breath and ventilator exhaust valves).
2. Press the Param. softkey and select the EtCO2
menu item, then press Enter.
3. Press the Zero softkey.
The unit zeroes the adapter and displays the
ZEROING CO2 ADAPTER message for 15 to 20
seconds.
The unit displays the message ZERO DONE upon
completion of the zeroing.
Note Do not attempt zeroing for 20 seconds after
removing the adapter from the patient’s airway.
This time allows any CO2 remaining in the adapter
to dissipate before zeroing. Do not attempt to zero
the adapter while it is connected to the patient’s
airway. Zeroing with CO2 in the adapter can lead to
inaccurate measurement and/or other error
conditions. If you attempt zeroing while CO2
remains in the adapter, the time required to zero
the adapter may be increased. If zeroing cannot be
completed, the message ZERO FAILED will be
displayed. If this occurs, clear any occlusion in the
adapter, remove any source of CO2, wait 20
seconds, and try zeroing again.
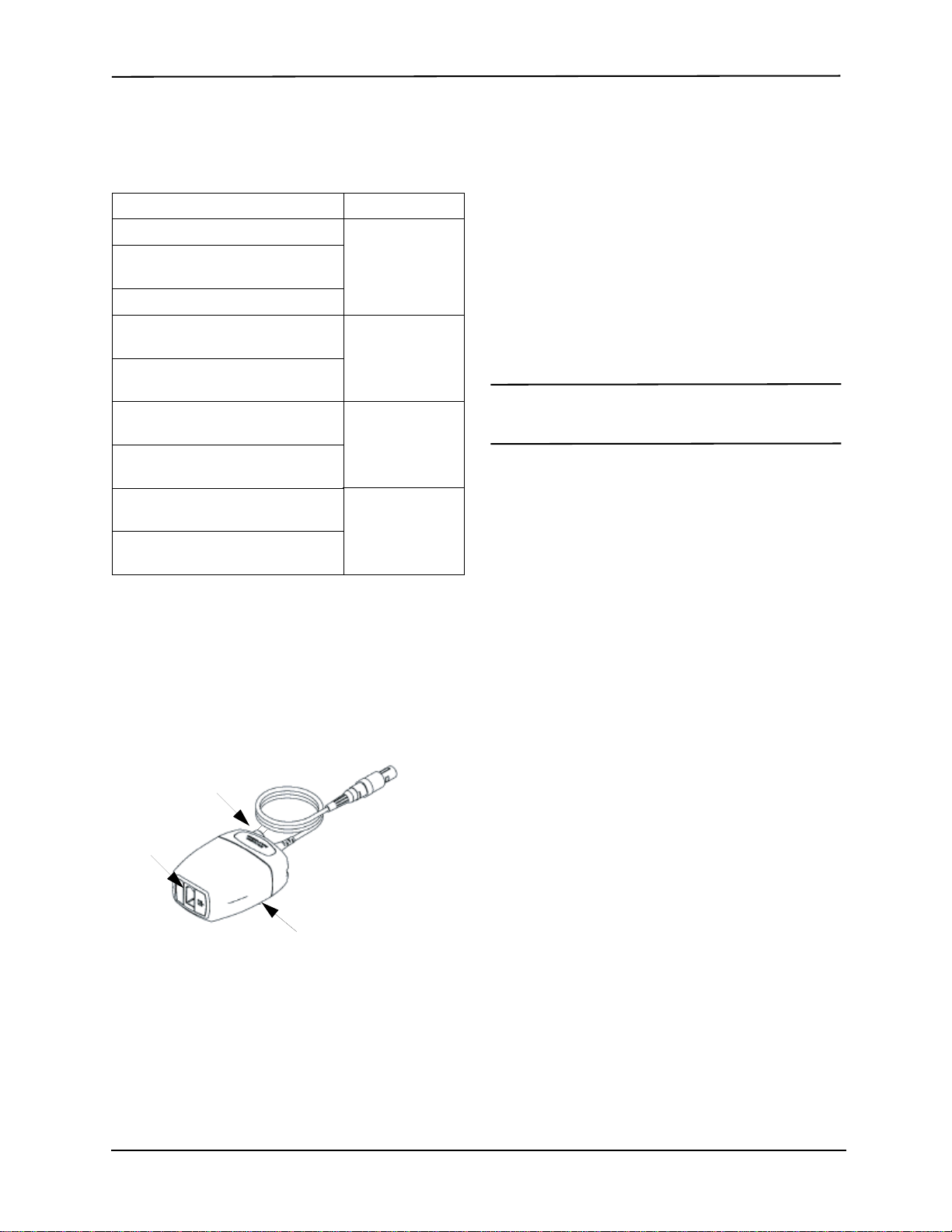
Attaching the Airway Adapter to the Airway
Circuit
If you have not yet done so, you must attach the airway
adapter to the CAPNOSTAT 5 CO2 sensor before
attaching the airway adapter to the airway circuit. Refer
to “Attaching the Airway Adapter to the CAPNOSTAT 5
CO2 Sensor” on page 4 if necessary.
Attach the airway adapter to the airway circuit as follows:
1. Place the CAPNOSTAT 5 CO2 sensor/airway adapter
assembly at the proximal end of the airway circuit
between the elbow and the ventilator circuit wye. Do
NOT place the airway adapter between the ET tube
and the elbow, as this may allow patient secretions to
accumulate in the adapter.
Position the airway adapter with its windows in a
vertical, NOT a horizontal, position. This helps keep
patient secretions from pooling on the windows. If
pooling does occur, the airway adapter may be
removed from the circuit, rinsed with water and
reinserted into the circuit. To prevent moisture from
draining into the airway adapter, do NOT place the
airway adapter in a gravity dependent position. See
Figure 2.
2. Check that connections have been made correctly by
verifying the presence of a proper CO2 waveform on
the E Series display.
3. The sensor cable should face away from the patient.
Figure 2
CAPNOSTAT
CO
2
Sensor
To Patient
Reusable Adult
Airway Adapter
Ventilator Wye