3
Safe handling and preparation
CAUTION
Federal law restricts this device to sale by, or on
order of a physician!
Note
A specially designed Aesculap endoscope is offered for
the MINOP InVent trocar system. For reasons of com-
patibility, the MINOP InVent trocars may only be used
with this special Aesculap endoscope.
►Ensure that the product and its accessories are
operated and used only by persons with the requi-
site training, knowledge, or experience.
►Read, follow, and keep the instructions for use.
►Use the product only in accordance with its
intended use, see Intended use.
►Remove the transport packaging and clean the new
product, either manually or mechanically, prior to
its initial sterilization.
►Store any new or unused products in a dry, clean,
and safe place.
►Prior to each use, inspect the product for loose,
bent, broken, cracked, worn, or fractured compo-
nents.
►Do not use the product if it is damaged or defec-
tive. Set aside the product if it is damaged.
►Replace any damaged components immediately
with original spare parts.
►Perform a function check of the irrigation channel
before each use.
►Perform a function check of the endoscope's stop
mechanism before each use.
►To avoid burns when using the MINOP InVent tro-
car in combination with HF electrodes, ensure that
HF current is activated only under visual control.
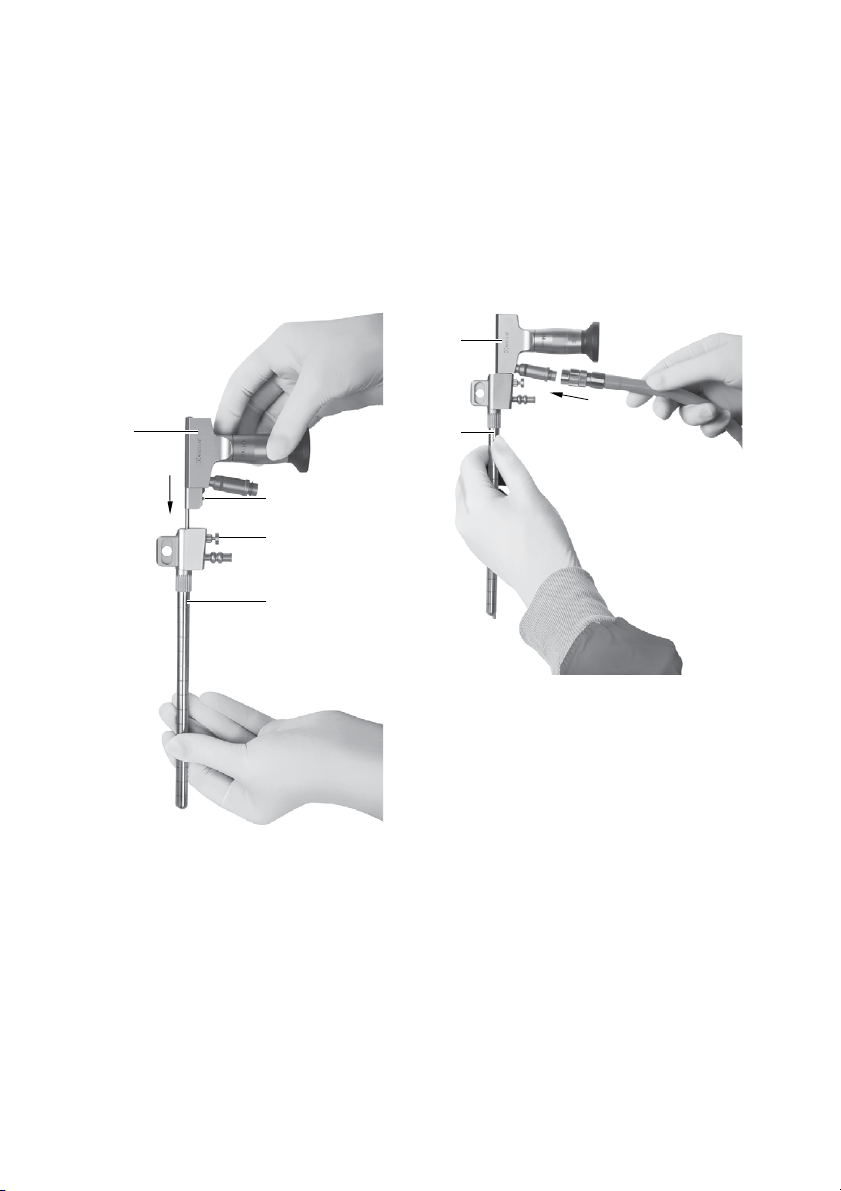
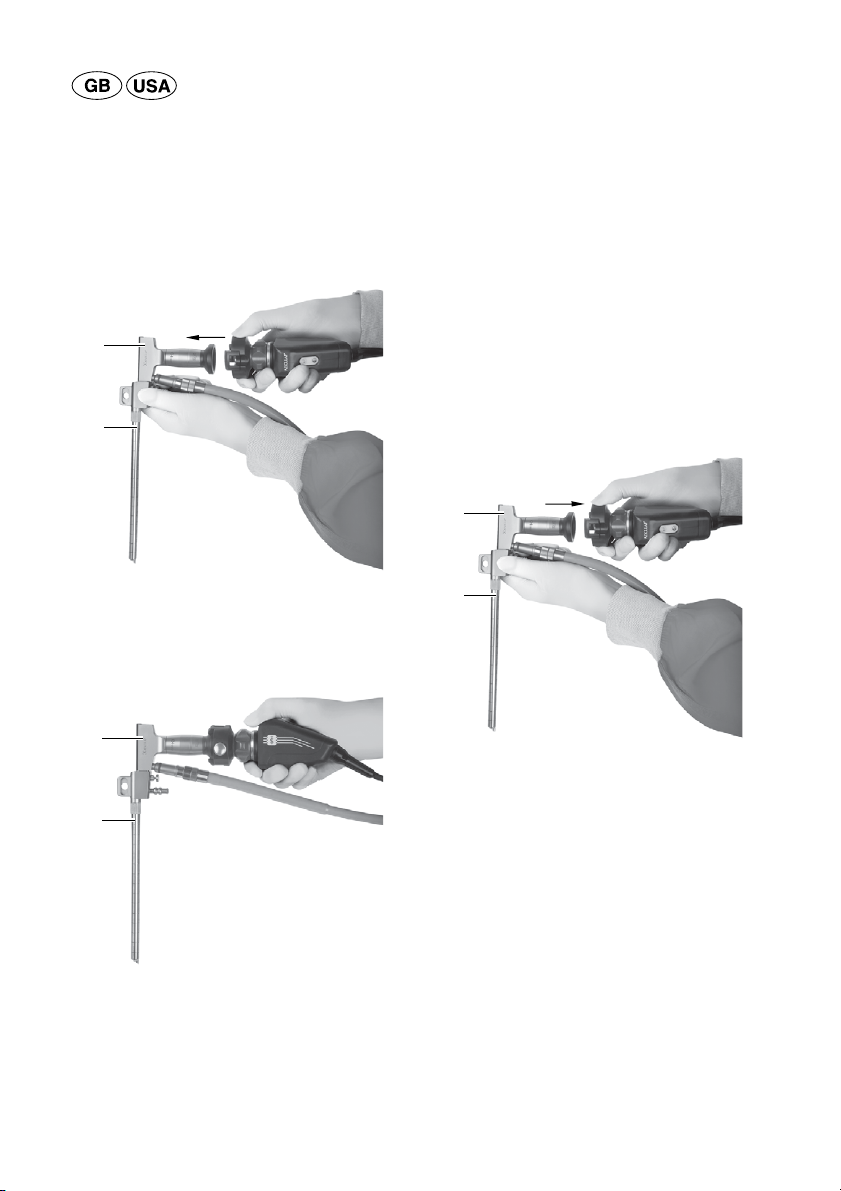
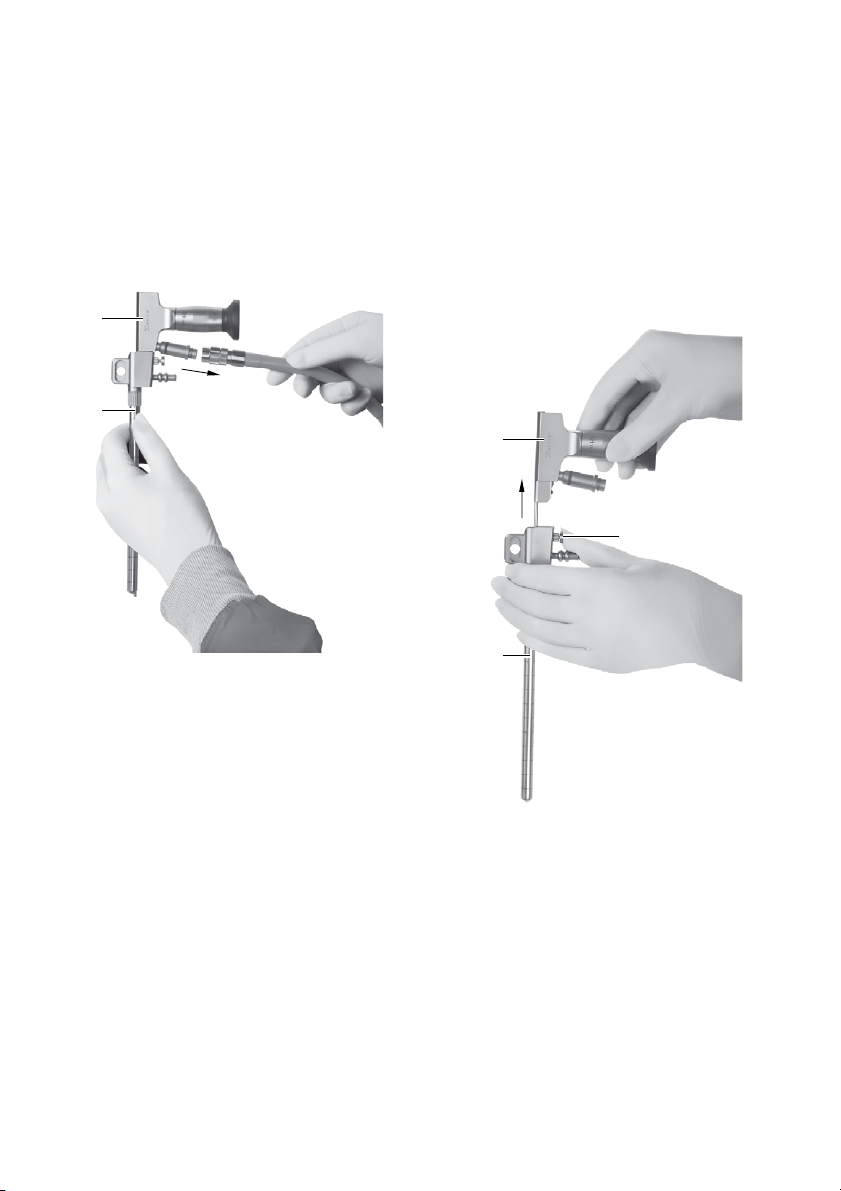
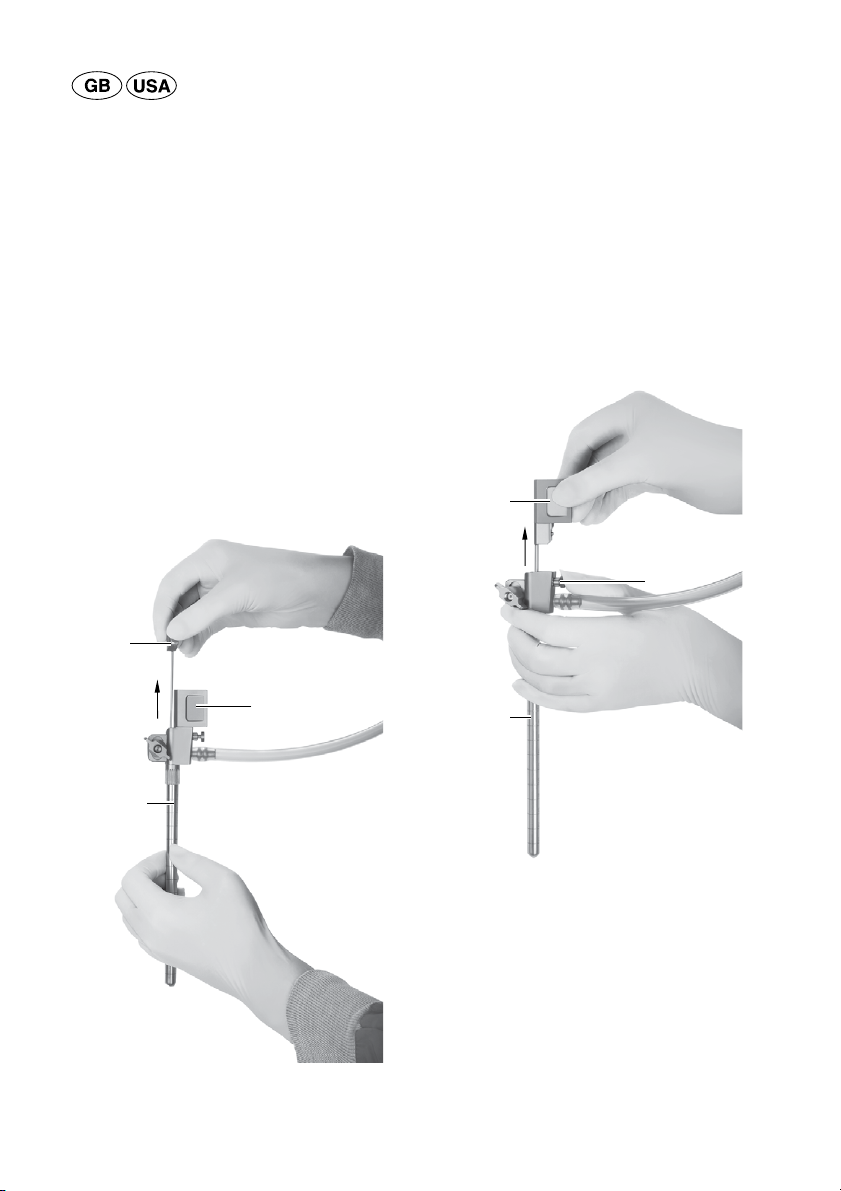
►When inserting the trocar into the brain/the ven-
tricle, close the working channel with the obtura-
tor provided.
Safe operation
WARNING
Risk of injury caused by incor-
rect operation of the product!
►Attend appropriate prod-
uct training before using
the product.
►For information about
product training, please
contact your national
B. Braun/Aesculap agency. WARNING
Risk of injury and/or malfunc-
tion!
►Always carry out a func-
tion check prior to using
the product.
WARNING
Risk of injury when using the
product beyond the field of
view!
►Apply the product only
under visual control.
WARNING
Risk of injury caused by pro-
truding 30° endoscope in
locked position!
►Insert the endoscope only
when the trocar is in its
final position.
WARNING
Risk of burns due to high tem-
perature of the instrument-
side end of the optical cable of
a light source!
►Apply proper care when
operating the light source.