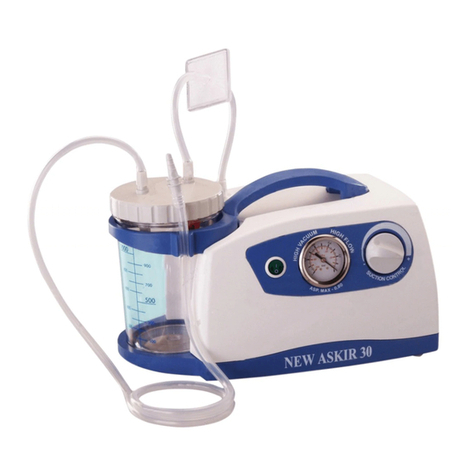
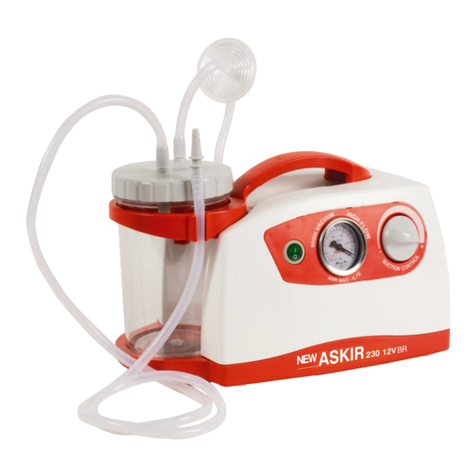
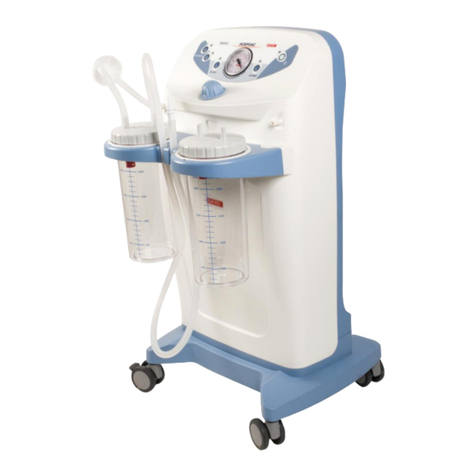
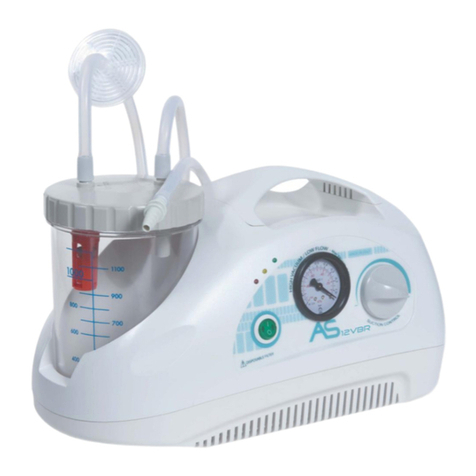
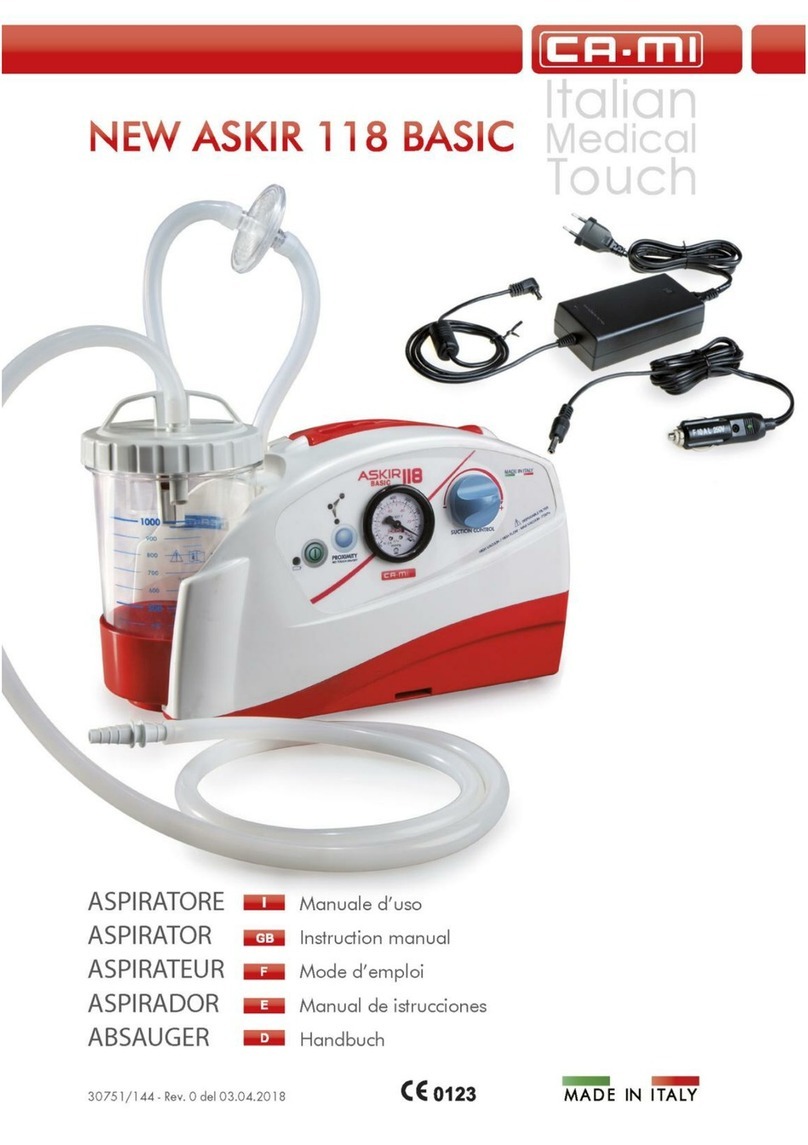
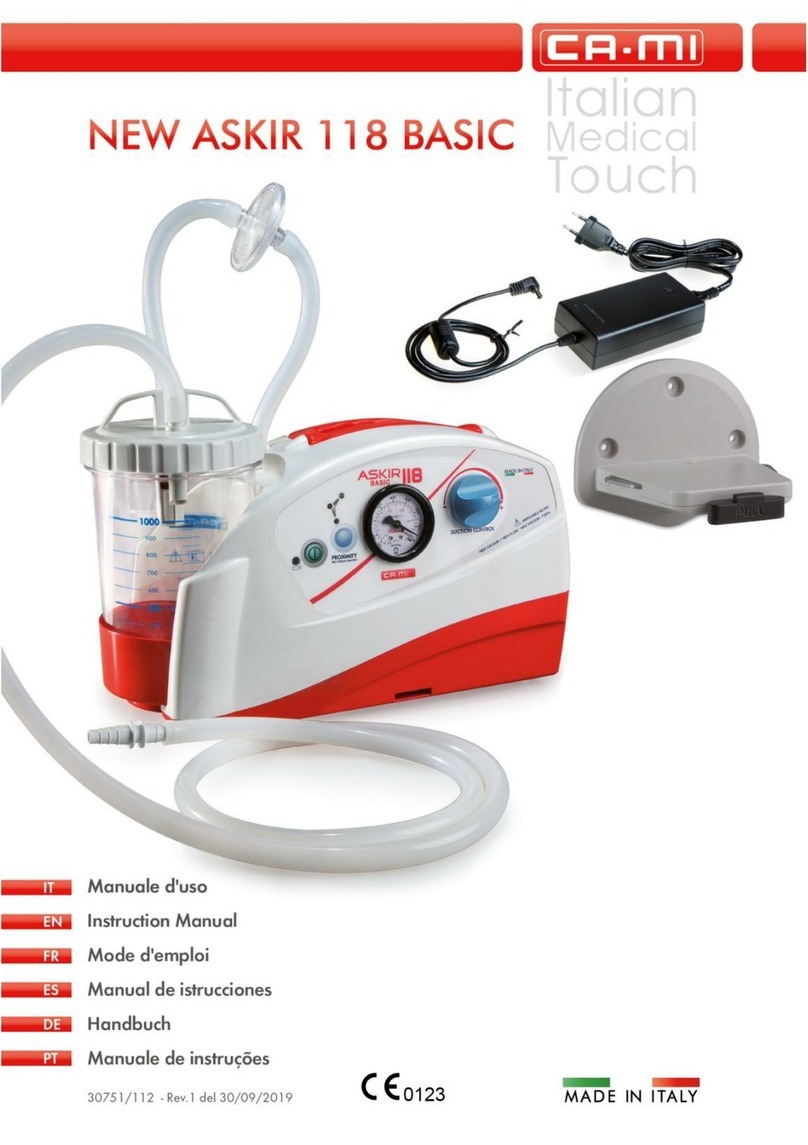
NEW ASKIR 30 Surgical aspirator is a portable unit, working with 230V ~ / 50 Hz network electricity, designed for the aspiration of bodily
fluids in adult and children. It’s particularly suitable for nasal, oral or tracheal aspiration of mucus, catarrh or blood after minor surgical
procedures and can be used in post-operative therapy at home or conveniently transported from one hospital ward to another.
Easily portable equipment designed for continuous use.
Made of highly heat resistant, electrically insulated plastic material in conformity with the latest European
safety standard, the product is supplied with a complete polycarbonate autoclavable jar with overflow valve and it is equipped with aspiration
regulator and vacuum indicator located on the front panel.
READ INSTRUCTION MANUAL CAREFULLY BEFORE USE
ONLY HIGHLY QUALIFIED STAFF USE RESERVED
THE INSTRUMENT MUST NOT BE DISASSEMBLED. FOR A TECHNICAL SERVICE ALWAYS CONTACT CA-MI
1. Check the condition of the unit before each use. The surface of the unit should carefully inspected for visual damage.
Check the mains cable and do not connect to power if damage is apparent;
2. Before connecting the appliance always check that the electric data indicated on the data label and the type of plug used, correspond to
those of the mains electricity to witch it’s to be connected;
3. If the plug supplied with the appliance is incompatible with the mains electricity socket, contact qualified staff for replacement of the
plug with a suitable type. The use of simple or multiple and / or extension adapters is not generally recommended.
Whenever their use is indispensable, use those in compliance with safety regulations, however paying attention not to exceed the
maximum power supply limits, which are indicated on the adapters and extensions;
4. Respect the safety regulations indicated for electrical appliances and particularly:
•Use original components and accessories provided by the manufacturer CA-MI to guarantee the highest efficiency and safety of
the device;
•The device can be used only with the bacteriological filter;
•Never immerge the appliance into water;
•Place instrument on stable and flat surfaces;
•Position the device in a way that the air inlets on the back aren’t obstructed;
•Don’t use in the presence of inflammable substances such as anaesthetic, oxygen or nitrous oxide;
•Don’t touch the device with wet hands and always prevent the appliance coming into contact with liquids;
•Keep off the reach of children or not capable people without supervision;
•Don’t leave the appliance connected to the power supply socket when not in use;
•Don’t pull the power supply cable to disconnect the plug remove the plug from the mains socket correctly;
•Preserve and use the medical device in environments protected from atmospheric factors and at a distance from heat sources;
•Don’t use the device thoracic drainage.
5. For repairs, exclusively contact CA-MI technical service and request the use of original spare parts.
Failure to comply with the above can jeopardise the safety of the device;
6. This medica device must be destined exc usive y for the use for witch it has been designed ad described in this manua .
Any different use must be considered incorrect and therefore dangerous; the manufacturer will not be responsible for damage due to
improper use or connection to an electrical system not complying with current regulations;
7. Particular precautions must be made concerning electromagnetic compatibility. The medical device must be installed and used
according to information supplied with the accompanying documents;
8. Instrument and accessories discharging must be done following current law regulations in every country of use.
9. None of electric or mechanical parts have been designed to be repaired by customers or end-users. Don’t open the device, do not
mishandle the electric / mechanical parts. Always contact CA-MI technical assistance
10. Using the device in environmental conditions different than those indicated in this manual may harm seriously the safety and the
technical characteristics of the same.
IMPORTANT INFORMATION FOR CORRECT DISPOSAL OF THE PRODUCT IN ACCORDANCE WITH EC DIRECTIVE 2002/96/EC:
In respect of art. 13 Decreto Legislativo 25 Luglio 2005, n.151 “Actuation of European directives 2002/95/EC, 2002/96/EC and 2003/108/EC, for
reduction in use of dangerous substances in the electric and electronic device and for garbage disposal”
The symbol as over applied on the device or its packaging means that at the end of its useful life the product must not be disposed of with domestic
waste. At the end of device useful, the user will must deliver it to the able collecting centres for electric and electronic garbage, or give back to the
retailer in the moment of equivalent new device purchasing, one against one. Disposing of the product separately prevents possible negative
consequences for the environment and for health, deriving from inadequate disposal. It also allows the recovery of materials of witch it’s made up in
order to obtain an important saving of energy and resources and to avoid negative effects to the ambient and health. In case of abusive disposal of
device by user, will be applied administrative endorsements in compliance with current standard.