Starkey Aspect User manual
Other Starkey Hearing Aid manuals

Starkey
Starkey BTE 13 User manual

Starkey
Starkey Evolv AI CIC User manual

Starkey
Starkey Livio AI User manual

Starkey
Starkey Livio User manual

Starkey
Starkey Rechargeable BTE User manual

Starkey
Starkey Genesis AI User manual
Starkey
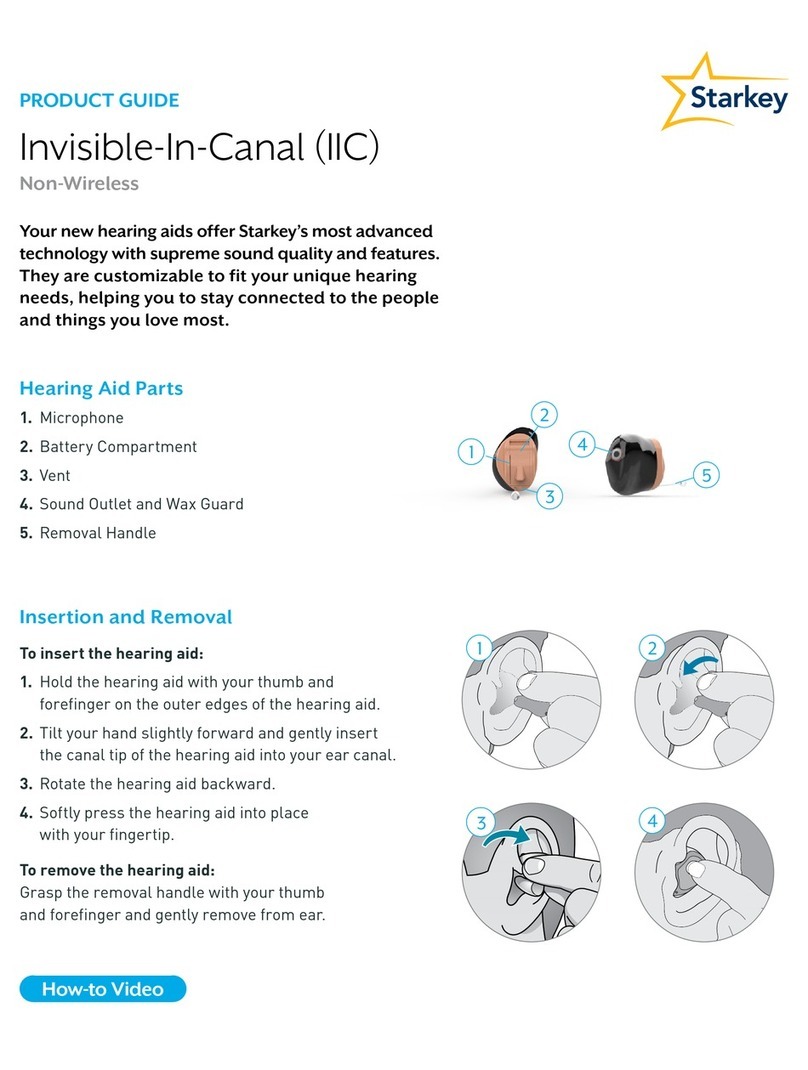
Starkey IIC User manual

Starkey
Starkey Livio BTE R User manual

Starkey
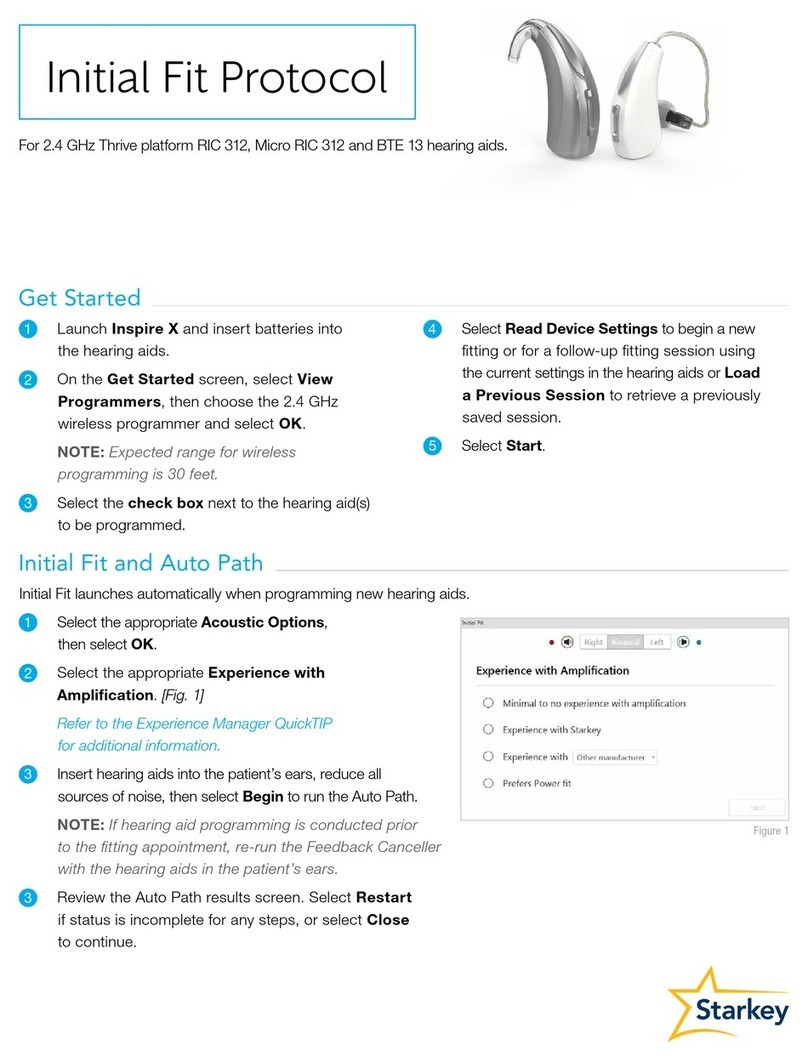
Starkey IIC NW User manual

Starkey
Starkey S Series iQ User manual

Starkey
Starkey IIC NW User manual

Starkey
Starkey RIC 10 User manual

Starkey
Starkey RIC 10 User manual

Starkey
Starkey Livio RIC R User manual

Starkey
Starkey ITE User manual

Starkey
Starkey RIC 312 User manual

Starkey
Starkey BTE R User manual

Starkey
Starkey Livio AI User manual
Starkey
Starkey RIC 312 User manual

Starkey
Starkey ARIES PLUS User manual
Popular Hearing Aid manuals by other brands
Oticon Medical
Oticon Medical Ponto SoundConnector Instructions for use
Siemens
Siemens Life micon user guide
Audicus
Audicus The Icon instruction manual

Advanced Bionics
Advanced Bionics Slim HP Standard Instructions for use

Widex
Widex DREAM440 THE DREAM SERIES User instructions

Phonak
Phonak myPilot user guide
























