Zeiss ELYRA User manual
Other Zeiss Laboratory Equipment manuals

Zeiss
Zeiss Auto Focus User manual

Zeiss
Zeiss TIRF 3 User manual

Zeiss
Zeiss ULTRAPHOT II User manual

Zeiss
Zeiss Axiocam 208 color User manual

Zeiss
Zeiss AT.Shooter A2-2000 User manual

Zeiss
Zeiss 200-mm airlock User manual

Zeiss
Zeiss Axio Imager A1 User manual

Zeiss
Zeiss Corona process User manual

Zeiss
Zeiss WSB ZPiezo CAN User manual

Zeiss
Zeiss ApoTome.2 User manual

Zeiss
Zeiss Axiocam 208 color User manual

Zeiss
Zeiss MCU 2008 User manual

Zeiss
Zeiss AxioObserver User manual

Zeiss
Zeiss Lightsheet 7 User manual

Zeiss
Zeiss HBO 50 User manual

Zeiss
Zeiss DirectFRAP User manual

Zeiss
Zeiss AxioObserver User manual

Zeiss
Zeiss METROTOM User manual

Zeiss
Zeiss Argon Ion Beam System User manual
Zeiss
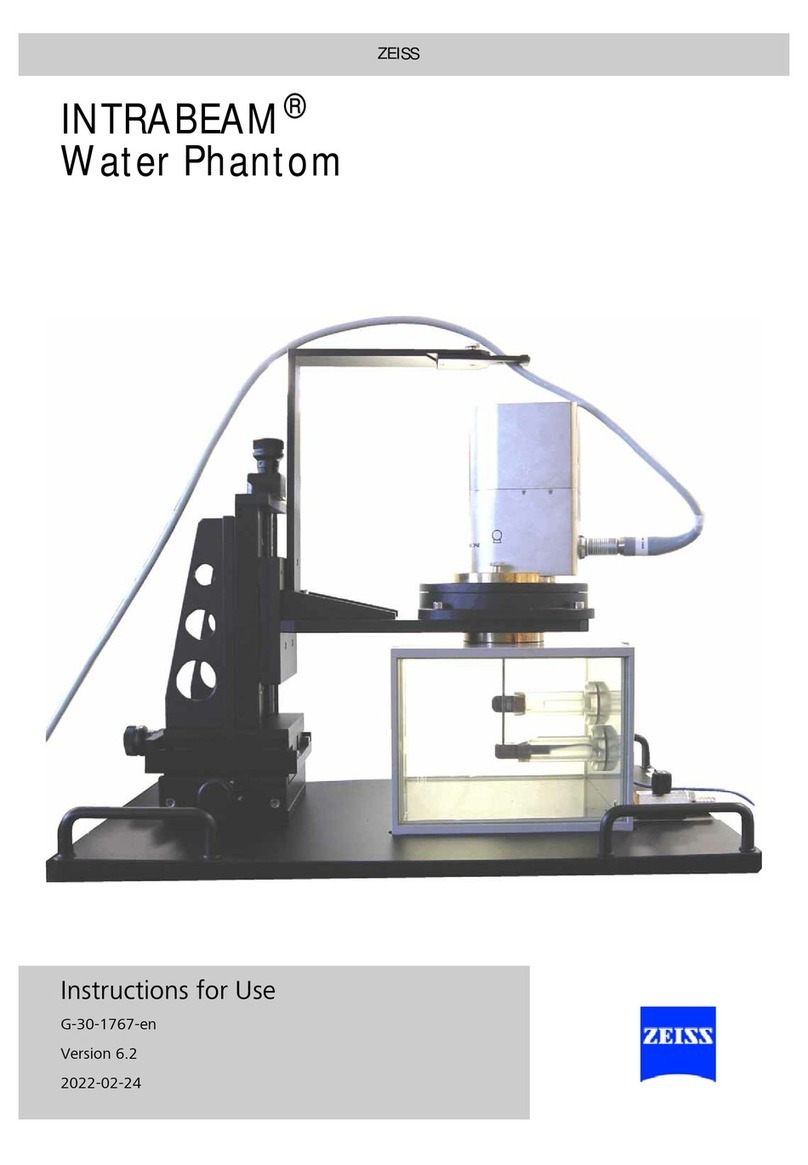
Zeiss INTRABEAM User manual
Popular Laboratory Equipment manuals by other brands

Belden
Belden HIRSCHMANN RPI-P1-4PoE installation manual

Koehler
Koehler K1223 Series Operation and instruction manual

Globe Scientific
Globe Scientific GCM-12 quick start guide

Getinge
Getinge 86 SERIES Technical manual

CORNING
CORNING Everon 6000 user manual

Biocomp
Biocomp GRADIENT MASTER 108 operating manual