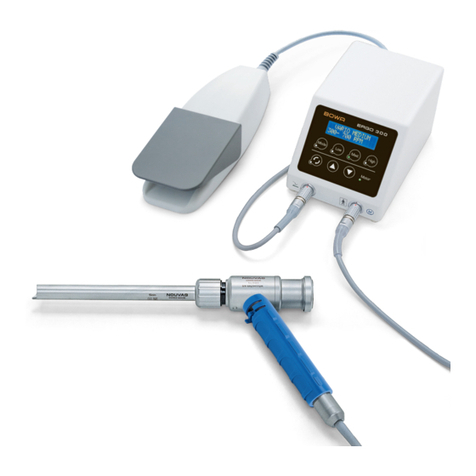
Bowa ERGOeco User manual

13120EN-S0
Instructions for Use
ERGOeco
en


Key
BOWA-IFU-13120-ERGOECO-S0-EN-20220420 Instructions for Use ERGOeco 1
Key
Handle
Rotation wheel
Shaft tube
Erbe plug
Release button for disassembly
Insert
with Jaw
Maryland insert
Kelly insert
Fenestrated insert

Key
2 Instructions for Use ERGOeco BOWA-IFU-13120-ERGOECO-S0-EN-20220420
Handle with plug versions
Shaft tube
2-pin 28 mm
2-pin 22mm
Erbe

Contents
BOWA-IFU-13120-ERGOECO-S0-EN-20220420 Instructions for Use ERGOeco 3
Contents
Key .................................................................................................................... 1
Contents ........................................................................................................... 3
1 Using the Instructions for use ....................................................................... 4
1.1 Scope of validity .....................................................................................................4
1.1.1 Kits ..................................................................................................................5
1.1.2 Spare parts ......................................................................................................5
1.2 Symbols and markings ...........................................................................................6
2 Purpose ......................................................................................................... 7
2.1 Indications ..............................................................................................................7
2.2 Contraindications ...................................................................................................7
3 Safety instructions ......................................................................................... 8
3.1 Device-related ........................................................................................................8
3.2 Use-related .............................................................................................................9
3.2.1 Patients with pacemakers ...............................................................................10
3.3 EMC information ....................................................................................................10
4 Mode of operation ......................................................................................... 11
5 Procedure during use .................................................................................... 11
6 Assembly ....................................................................................................... 12
7 Operation ...................................................................................................... 13
7.1 Before use ..............................................................................................................13
7.2 Function test in the operating theatre ...................................................................13
7.3 During the surgery .................................................................................................14
7.4 Removal ..................................................................................................................15
7.5 After use .................................................................................................................15
7.5.1 Spare parts .....................................................................................................15
8 Disassembly ................................................................................................... 16
9 Reprocessing ................................................................................................. 17
9.1 General information ...............................................................................................17
9.2 Device-specific information ....................................................................................17
9.3 Check for damage ..................................................................................................19
9.4 Sterilisation packaging ...........................................................................................20
10 Ambient conditions ..................................................................................... 20
10.1 Storage and transport ..........................................................................................20
11 Technical data .............................................................................................. 21
12 Symbols on packaging ................................................................................. 21

Using the Instructions for use
4 Instructions for Use ERGOeco BOWA-IFU-13120-ERGOECO-S0-EN-20220420
1 Using the Instructions for use
These Instructions for use are part of the device.
BOWA-electronic GmbH & Co. KG assumes no liability and provides no warranty
whatsoever for any damage or consequential damage arising
from non-compliance with these Instructions for use.
Read the Instructions for use, in particular the Safety chapter (see chapter 3 “Safety
instructions”), carefully and thoroughly before use.
Keep the Instructions for use in a safe place throughout the service life of the device.
Keep the Instructions for use accessible to operating theatre personnel.
Hand the Instructions for use to any subsequent owner or user of this device.
Always update the Instructions for use whenever you receive additional information
from the manufacturer.
1.1 Scope of validity
These Instructions for use apply only to the devices specified in chapters 1.1.1 and 1.1.2.

Using the Instructions for use
BOWA-IFU-13120-ERGOECO-S0-EN-20220420 Instructions for Use ERGOeco 5
1.1.1 Kits
1.1.2 Spare parts
Name
Kit
Connector type /
Handle Insert REF
360 mm 460 mm
ERGOeco, Maryland,
for Erbe
Erbe
Maryland 770-670 770-671
ERGOeco, Kelly,
for Erbe Kelly 770-672 770-673
ERGOeco,fenestrated,
for Erbe fenestrated 770-674 770-675
ERGOeco, Maryland,
for 2-pin, 22 mm
2-pin 22mm
Maryland 770-680 770-681
ERGOeco, Kelly,
for 2-pin 22 mm Kelly 770-682 770-683
ERGOeco,fenestrated,
for 2-pin 22 mm fenestrated 770-684 770-685
ERGOeco, Maryland,
for 2-pin 28 mm
2-pin 28mm
Maryland 770-690 770-691
ERGOeco, Kelly,
for 2-pin 28 mm Kelly 770-692 770-693
ERGOeco,fenestrated,
for 2-pin 28 mm fenestrated 770-694 770-695
REF
Name of spare part 360 mm 460 mm
ERGOeco, handle for Erbe, cable 4.5 m 770-605
ERGOeco, handle for 2-pin 22 mm, cable 4.5 m 770-606
ERGOeco, handle for 2-pin 28 mm, cable 4.5 m 770-607
ERGOact / ERGOeco, shaft tube, Ø 5 mm 770-610 770-620
ERGOact / ERGOeco, Insert Maryland 770-611 770-621
ERGOact / ERGOeco, Insert Kelly 770-612 770-622
ERGOact / ERGOeco, Insert fenestrated 770-613 770-623

Using the Instructions for use
6 Instructions for Use ERGOeco BOWA-IFU-13120-ERGOECO-S0-EN-20220420
1.2 Symbols and markings
Level of warning notices:
Danger levels in warning notices:
Tips:
SIGNAL WORD
Type, source and consequences of the hazard (personal injuries)!
Measure for preventing the hazard.
Symbol Danger level Probability of occurrence Consequences of
non-compliance
HAZARD Immediate hazard Death, severe injury
WARNING Possible hazard Death, severe injury
CAUTION Possible hazard Minor injury
NOTICE Possible hazard Property damage
Tips to make your work easier or supplementary information explaining
a work procedure.

Purpose
BOWA-IFU-13120-ERGOECO-S0-EN-20220420 Instructions for Use ERGOeco 7
Other symbols and markings:
2 Purpose
Electrosurgical equipment for tissue cutting and coagulation.
2.1 Indications
The ERGOeco instruments are designed for the grasping, bipolar coagulation and
dissection of biological tissue during minimally invasive surgery.
2.2 Contraindications
The medical device must not be used if, in the opinion of an experienced physician or
according to the current technical literature, such usage could endanger either the
patient, e.g. due to their general condition, or other persons.
Do not use the medical device if its surgical techniques are contraindicated.
Never use the medical device on the heart, central circulatory system or central nervous
system.
Symbol / marking Definition
Prerequisite for an activity
Activity with one step
1.
2. Activity with several steps in strict sequence
Result of preceding activity
• List (first level)
• List (second level)
Emphasis Emphasis

Safety instructions
8 Instructions for Use ERGOeco BOWA-IFU-13120-ERGOECO-S0-EN-20220420
3 Safety instructions
3.1 Device-related
• The instrument may only be used by trained medical staff. The surgeon and the medical
professionals must be trained in, and hence be familiar with, the fundamental principles,
codes of practice and risks of HF surgery.
Read the instructions for use carefully and thoroughly before using the device.
All serious incidents occurring in connection with the device must be reported to the
manufacturer and to the competent authority of the user’s country of residence.
• The devices are supplied in non-sterile condition and must be cleaned and sterilised
before use.
• Risk of injury from sharp edges.
• The device must not be operated in AUTOSTART mode.
• You are responsible for ensuring the instruments are sterile
• Please comply with the following:
Always clean and sterilise the handle and the corresponding insert
before each use (see chapter 9 “Reprocessing”).
Only use cleaning, disinfection and sterilisation methods that have been validated for
the specific devices and products concerned.
Comply with the validated parameters for each cycle.
Comply with the legal regulations applicable in your country, as well as the
hygiene regulations of the hospital.
Do not use gas sterilisation or hot air sterilisation as these methods can damage the
device.
• To avoid burns (thermal overload), please comply with the following:
Make sure that all electrical plug connections are correctly seated.
Do not strain the devices beyond their mechanical limits
(e.g. by excessively bending, kinking, or crushing them or by running them over with
an instrument trolley).
The maximum rated voltage of the instrument must not be exceeded (see chapter 11
“Technical data”).
Comply with the recommended power settings and maximum voltage of the
designated HF generator.
The effectiveness of the settings must be assessed by the user.
• In order to rule out the risk of injuries and electric shocks for the patient and the operating
personnel, make sure that the power supply is switched off before connecting the HF
connection cable and accessories to and removing them from the electrosurgical device.
• Cables must never be inserted or removed while the device is activated.
• Do not repair or service defective devices:
Check the device for damage, in particular for the integrity of the insulation, after
reprocessing and before use.
Replace and dispose of defective devices immediately.

Safety instructions
BOWA-IFU-13120-ERGOECO-S0-EN-20220420 Instructions for Use ERGOeco 9
Comply with the Instructions for use of the HF device and the general instructions for
electrosurgical operations!
• If necessary, use a suitable tester (e.g. BOWA REF 050-230) to check if the device is in
proper working order.
• The "BF" / "CF" applied part of the HF device used is extended by the instrument
connected to it.
3.2 Use-related
• Make sure that the HF generator you intend to use is connected to a matching instrument
handle (see chapter 1.1 “Scope of validity”).
• Before you use the device, run a function test using a gauze soaked in saline solution.
• Only insert the bipolar instrument into the body if you have a clear field of vision.
• Ensure that you have adequate visibility before activating the jaws .
• For coagulation in the vicinity of sensitive structures, such as nerves or the ureter,
proceed with the utmost caution .
• The jaws must not be too full. Never grasp too much tissue for coagulation.
• Improper use of HF current can lead to burns and explosions:
Perform electrosurgical operations only with insufflation of non-flammable gases
(CO2).
Avoid direct skin contact with HF cables.
Avoid contact with flammable gases and liquids.
• Improper use of the device can cause injury to the patient:
Avoid skin-to-skin contact between the HF cable and the patient.
When plugging in or removing the HF cable, always take hold of the connector, never
pull on the cable itself.
To prevent injury due to inadvertant activation of the HF,do not place the instrument
on the patient.
Avoid contact with metallic objects (clips, stents, etc.) in the vicinity of the active jaws.
This can affect energy output and lead to adverse effects.
• The jaws can get hot enough to cause a thermal lesion.
• The HF cable may cause interference to imagery on monitors:
Never route the HF cable alongside a camera cable.
Do not lay the HF cable in loops.
Consult the Instructions for use of the BOWA HF generators and other compatible
generators for additional information on possible interference with other devices.
• Electrical safety is improved through the use of plastic trocars.

Safety instructions
10 Instructions for Use ERGOeco BOWA-IFU-13120-ERGOECO-S0-EN-20220420
3.2.1 Patients with pacemakers
Pacemaker malfunction or damage to the pacemaker can endanger the life of the patient
or result in irreversible injuries to the patient.
If you are treating a patient with a pacemaker, consult the cardiologist first before
performing HF surgery.
Set the pacemaker to a fixed demand frequency.
Make sure that the pacemaker does not directly contact the jaws.
Have a functioning defibrillator on hand.
Carry out a postoperative pacemaker check.
3.3 EMC information
Since medical electrical devices are subject to special EMC precautions, please comply
with the following information.
The BOWA accessory is only intended to be connected to BOWA-specified HF devices.
Using the accessory with medical devices from other manufacturers may result in
higher emission levels or reduced interference immunity.
NOTICE
Combining medical devices is safe only if
the lowest power rating (rated voltage/type of current, ...) is used for
all devices in the desired combination(s).
doing so is permitted according to the purpose and by the interface
specification of the devices used in the combination.
The Instructions for use and interface specifications of medical
devices used in combination must be strictly observed.

Mode of operation
BOWA-IFU-13120-ERGOECO-S0-EN-20220420 Instructions for Use ERGOeco 11
4 Mode of operation
In bipolar HF surgery, tissue coagulation is achieved by applying a high-frequency AC
current, which generates heat.
The ERGOeco bipolar coagulation instrument is a surgical instrument for use in
laparoscopic surgery.
It is used through surgical incisions in conjunction with devices for endoscopic use, such
as trocars and optics.
The active jaws are the uninsulated areas of the insert. The HF current flows from one jaw
of the instrument through the biological tissue to the second jaw to produce the desired
localised tissue effect.
The insert can be rotated and positioned by a rotary wheel on the handle.
5 Procedure during use
1. Prior to use, clean and sterilise the new product that is shipped in non-sterile condition
from the factory.
2. Check to make sure that all individual parts are present and mount the instrument (see
chapter 6 “Assembly”).
3. Connect the HF generator you plan to use to a matching instrument handle (see
chapter 1.1 “Scope of validity”).
4. Do not exceed the maximum permissible voltage (see chapter 11 “Technical data”).
5. Always perform a thorough visual inspection and function check before use.
6. Insert the jaws, in the closed state, into the trocar sleeve.
7. Insert the bipolar instrument into the body whilst keeping visual contact.
8. Only perform the operation if you have clear visibility.
9. After the surgery, disconnect the instrument from the HF generator and disassemble it.
10. Reprocess the devices after each use.

Assembly
12 Instructions for Use ERGOeco BOWA-IFU-13120-ERGOECO-S0-EN-20220420
6 Assembly
Assemble the instrument in the following sequence:
1. Introduce the insert into the shaft tube.
2. Take hold of the jaw and carefully turn it to tighten it.
The shaft with insert is now ready to be mounted on the handle.
3. Whilst ensuring that the jaws are closed, introduce the shaft tube with insert into the
completely opened handle until you hear it click into place.
When the shaft with insert is aligned correctly and fully seated inside the handle,
moving the rear handle shank will cause the instrument’s jaws to move. The
instrument is now completely assembled.

Operation
BOWA-IFU-13120-ERGOECO-S0-EN-20220420 Instructions for Use ERGOeco 13
7 Operation
7.1 Before use
The instrument is now assembled (see chapter 6 “Assembly”).
1. Switch on the HF device and connect the HF cable to the HF device.
2. Set the required settings on the HF device.
3. Always perform a thorough visual inspection and function check before each use of the
laparoscopic instrument (see see chapter 9.3 “Check for damage”).
7.2 Function test in the operating theatre
1. Check whether the jaws can be easily opened and closed using the handle.
2. Check the activation by means of the foot switch.
The activation signal is emitted during activation of the foot switch.
WARNING
Risk of patient injury!
Only use compatible HF generators.
Select the settings of the HF device according to the requirements of
the intervention.
Only use suitable devices and accessories wherever possible.
Only use intact and sterilised devices.
WARNING
Risk of patient injuries from burns or explosion!
Avoid contact with flammable gases and liquids
(such as skin cleansers, disinfectants and anaesthetic gases).
Avoid direct skin contact with HF cables.
Skin-to-skin contact (e.g. between the patient's arms and body)
should be avoided, e.g. by inserting dry gauze.

Operation
14 Instructions for Use ERGOeco BOWA-IFU-13120-ERGOECO-S0-EN-20220420
7.3 During the surgery
Introducing the instrument
1. Close the handle in order to keep the jaws closed.
2. Insert the instrument into the trocar sleeve.
Grasping, clamping and sealing tissue
1. Position the jaws at the surgical site.
2. Turn the rotary wheel (with the jaws open) to align the jaws to the target tissue.
3. Place the tissue to be coagulated between the jaws.
4. Close the jaws to grasp the tissue. Never grasp too much tissue.
5. Use the foot switch to activate the HF current of the HF generator for coagulation:
A continuous acoustic signal is emitted during the entire activation period.
WARNING
Risk of patient injury due to tissue pinching, especially with limited visibility!
Expose the tissue to be coagulated as much as possible to prevent it from
becoming pinched inadvertently!
Only perform the operation if you have clear visibility.
WARNING
Risk of patient injuries from hot jaws and release of steam!
Instrument tips may still be hot immediately after HF power has been
switched off.
Maintain sufficient distance between instrument tips and sensitive
tissue structures, such as nerves, pancreas or intestines.
Do not use hot laparoscopy instruments to prepare the tissue.
Do not place any laparoscopy instrument on the patient.
WARNING
Risk of patient injury due to accidental activation of the laparoscopy instrument!
Never use the AUTOSTART function.
Do not switch on the HF current before the jaws are in contact with
the tissue to be coagulated.
Activating the instrument inadvertently can cause injuries to the patient.

Operation
BOWA-IFU-13120-ERGOECO-S0-EN-20220420 Instructions for Use ERGOeco 15
6. Release the switch.
The tissue is coagulated.
The tissue has been grasped by the jaws and is coagulated.
7.4 Removal
1. Close the jaws by closing the handle.
2. Withdraw the instrument from the trocar sleeve.
7.5 After use
7.5.1 Spare parts
For an overview of the available spare parts, please refer to chapter 1.1.2 “Spare parts”.
To order spare parts, contact your BOWA dealership or visit our website:
www.bowa-medical.com.
WARNING
Incomplete coagulation!
Never use the AUTOSTART function.
Do not switch on the HF current before the jaws are in contact with
the tissue to be coagulated and ensure that they are within the
surgical field of view.
Inadvertent activation of the instrument can cause injuries to the patient.
WARNING
Incomplete coagulation due to soiled jaws!
Regularly clean the jaws with a damp cloth or a sterile plastic brush
if tissue adheres to them.
WARNING
Risk of patient injury due to fractured or damaged parts!
Check the instrument after each use. All parts must be present.
WARNING
Defective, worn or soiled jaws can cause instrument malfunction!
Clean soiled jaws after use with a damp cloth or a sterile plastic
brush. See also “Device-specific information”, page 17.
Inserts with damaged jaws must be replaced.

Disassembly
16 Instructions for Use ERGOeco BOWA-IFU-13120-ERGOECO-S0-EN-20220420
8 Disassembly
To disassemble the instrument, proceed as follows:
1. Hold the rear handle shank to keep it open.
2. Press the black button on the top of the handle.
3. Release the shaft by pulling it out of the handle and make sure the rear handle shank
does not lower again.
You have now separated the shaft from the handle.
4. Gently rotate the insert until it detaches.
5. Pull the insert out of the shaft tube.
The instrument is now disassembled.
1.
2.
3.

Reprocessing
BOWA-IFU-13120-ERGOECO-S0-EN-20220420 Instructions for Use ERGOeco 17
9 Reprocessing
9.1 General information
The following values for the permitted number of reprocessing cycles are intended as a
guide. The actual number may vary depending on the demands placed on the devices.
Manual reprocessing methods are not recommended due to their significantly lower
effectiveness.
Follow the chemical manufacturers’ statements on concentrations and exposure times.
The medical device manufacturer shall not be held liable if other types of cleaning agents
and disinfectants are used.
9.2 Device-specific information
Insulated parts must not come into contact with hard, sharp or heavy devices, as this
could damage the electrical insulation.
CAUTION
Risk of infection from water splashes and steam during manual pre-
cleaning!
Wear a face mask and protective clothing.
Adequate ventilation is recommended.
NOTICE
Abrasives and metal brushes damage the jaws!
Never clean the laparoscopy instrument using abrasives or metal
brushes.
NOTICE
Compressed air causes damage to the instrument parts!
Restrict compressed air pressure to 3 bar or less for drying the
instrument parts.

Reprocessing
18 Instructions for Use ERGOeco BOWA-IFU-13120-ERGOECO-S0-EN-20220420
Reprocessing cycles 50 (depending on the intensity of use)
Reprocessing step Description
Manual pre-cleaning
1.A Soak1
Soak the dismantled device at room temperature (<25°C) for at least
5 minutes immediately, but no later than 2 hours, after use. Only use
enzymatic, aldehyde-free disinfectants suitable for disinfecting medical
devices (e.g., DGHM or FDA approval or CE marking). Remove all visible
soiling with a soft plastic brush. BOWA recommends the use of
Gigasept®Instru AF (Schülke & Mayr GmbH).
1.B Ultrasound 2
Place the individual parts of the device in the ultrasonic bath
for at least 5 minutes. BOWA recommends the use of Gigasept®Instru AF
(Schülke & Mayr GmbH).
2 Rinsing
Thoroughly rinse the individual parts of the device for at least 1 minute
at room temperature (<25°C) under running tap water (drinking water
quality or better). Allow residual water to drip off sufficiently.
Machine cleaning, thermal disinfection and drying
3 Machine
cleaning 3
For machine cleaning and disinfection, the shaft tube is inserted into a
rinsing sleeve and the handle is attached to an injector nozzle. The
opened insert is placed in a wire basket.
Make sure that the cable is not kinked or pinched.
Use a washing and disinfecting device (WD) with certified efficiency
(complying with ISO 15883) and a neutral to mildly alkaline (max.
pH 11.5) enzymatic cleaning agent without critical constituents.
Depending on the concentration, agents including alcohol- and/or
aldehyde-containing ingredients may be used.
BOWA recommends the use of neodisher®MediClean forte (Chemische
Fabrik Dr. Weigert) at 55°C for 10 minutes.
The intermediate rinse must comprise at least two rinsing steps using
demineralised water (each step taking 1 minute at >10°C).
4 Thermal
disinfection4
An A0value of >3000 must be attained.
BOWA recommends a temperature of 90°C for at least 5 minutes and
the use of demineralised water for this purpose.
5Drying
Drying is carried out according to the WD programme and depends on
the total load. The maximum temperature is 100°C for 25 minutes. If
necessary, dry with filtered compressed air at max. 3 bar.
Inspection
After machine cleaning and disinfection, carry out a visual inspection to
check for residues.
Repeat the reprocessing, if necessary.
Table of contents
Other Bowa Medical Equipment manuals

Bowa
Bowa SHE SHA User manual

Bowa
Bowa TissueSeal PLUS COMFORT Series User manual

Bowa
Bowa Lotus User manual

Bowa
Bowa 901-011 User manual

Bowa
Bowa ERGO 315R User manual

Bowa
Bowa ERGO 310D User manual

Bowa
Bowa ErgoLAP BIPOLAR User manual

Bowa
Bowa ARC 350 User manual

Bowa
Bowa ARC 400 User manual
Bowa
Bowa ERGO 300 Specification sheet
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual