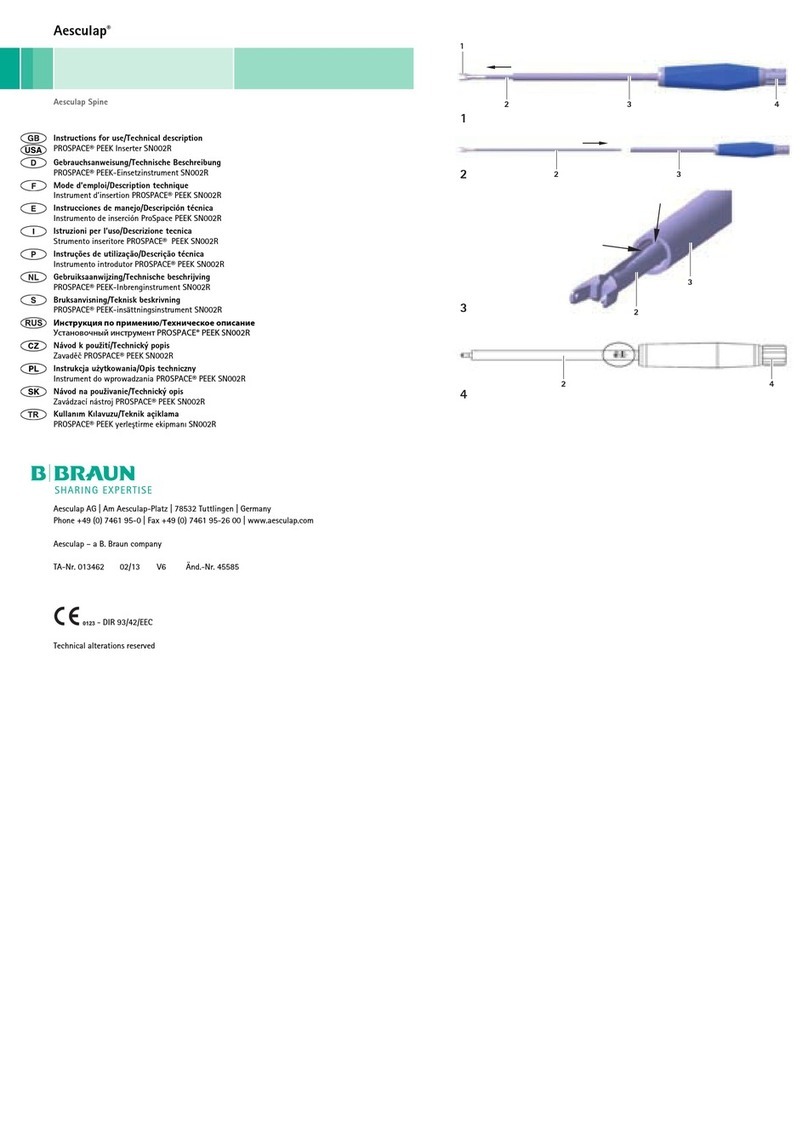
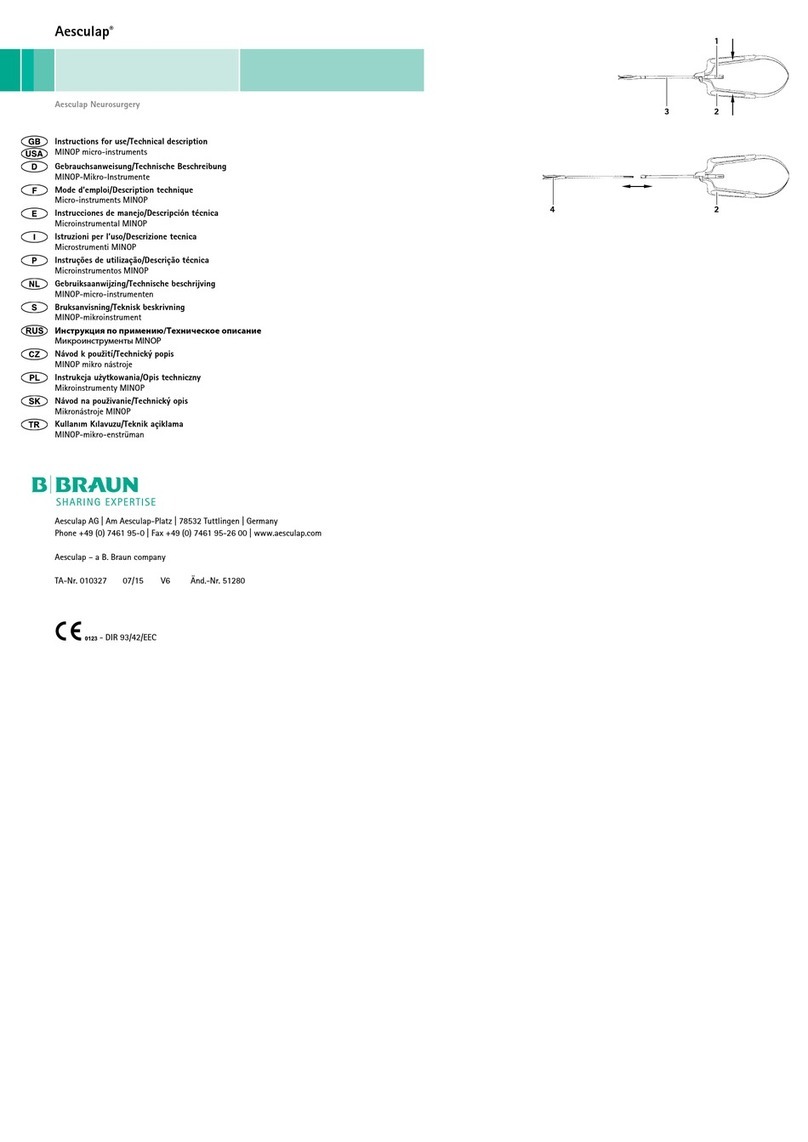
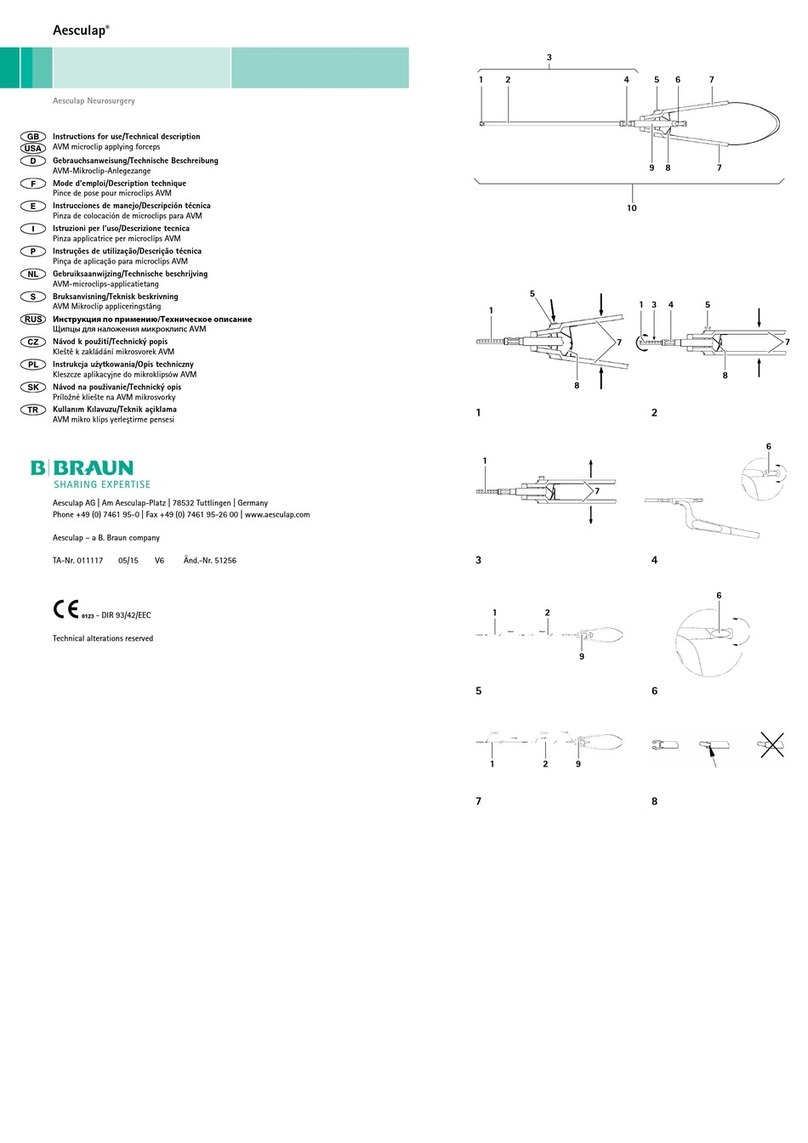
3
en
Contents
1. Applicable to . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
2. General information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
2.1 Intended use. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
2.2 Main functions and design characteristics . . . . . . . . . . . . . . . . 3
2.3 Indications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
2.4 Absolute contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
2.5 Relative contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
3. Safe handling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
4. Product description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
4.1 Scope of supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
4.2 Components required for operation. . . . . . . . . . . . . . . . . . . . . . 4
4.3 Operating principle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
5. Preparation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
6. Working with the device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
6.1 System set-up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
6.1.1 Connecting the accessories. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
6.1.2 Inserting the rechargeable battery. . . . . . . . . . . . . . . . . . . . . . . 5
6.1.3 Intraoperative battery change . . . . . . . . . . . . . . . . . . . . . . . . . . 5
6.1.4 Removing the rechargeable battery . . . . . . . . . . . . . . . . . . . . . . 5
6.1.5 Protection against inadvertent activation. . . . . . . . . . . . . . . . . 6
6.1.6 To mount the flap rod and flaps. . . . . . . . . . . . . . . . . . . . . . . . . 6
6.1.7 Inserting the dermatome blade . . . . . . . . . . . . . . . . . . . . . . . . . 6
6.1.8 Removing the dermatome blade. . . . . . . . . . . . . . . . . . . . . . . . . 6
6.1.9 Intraoperative storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
6.2 Function checks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
6.3 Safe operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
6.3.1 Adjusting the cutting thickness . . . . . . . . . . . . . . . . . . . . . . . . . 6
6.3.2 Adjusting the cutting width . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
6.3.3 Operating the product. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
6.3.4 Taking skin grafts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
7. Validated reprocessing procedure . . . . . . . . . . . . . . . . . . . . . . . 7
7.1 General safety notes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
7.2 General information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
7.3 Preparations at the place of use. . . . . . . . . . . . . . . . . . . . . . . . . 7
7.4 Disassembling the product before carrying out the reprocessing
procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
7.4.1 Removing the flap rod of the dermatome . . . . . . . . . . . . . . . . . 8
7.5 Preparation before cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
7.6 Single-use products. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
7.7 Cleaning/disinfection. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
7.7.1 Product-specific safety instructions for the reprocessing
procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
7.8 Manual cleaning with wipe disinfection . . . . . . . . . . . . . . . . . . 9
7.9 Automatic cleaning/disinfection with manual pre-cleaning . . 10
7.9.1 Manual pre-cleaning with a brush. . . . . . . . . . . . . . . . . . . . . . . 10
7.9.2 Mechanical alkaline cleaning and thermal disinfection. . . . . . 11
7.10 Inspection, maintenance and checks . . . . . . . . . . . . . . . . . . . . . 11
7.11 Packaging . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
7.12 Steam sterilization. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
7.13 Sterilization for the US market. . . . . . . . . . . . . . . . . . . . . . . . . . 12
7.14 Storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
8. Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
9. Troubleshooting list. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
10. Technical Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
11. Accessories/Spare parts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
12. Technical data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
12.1 Classification acc. to Directive 93/42/EEC . . . . . . . . . . . . . . . . 15
12.2 Performance data, information about standards . . . . . . . . . . . 15
12.3 Operating mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
12.4 Environmental conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
13. Disposal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
14. Distributor in the US/Contact in Canada for product
information and complaints. . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
1. Applicable to
►For item-specific instructions for use and information on material
compatibility, see also the Aesculap Extranet at
https://extranet.bbraun.com
2. General information
2.1 Intended use
Task/Function
The Dermatome GA340 / Dermatome 0.1 mm GA341, combined with der-
matome blade, is used for split skin removal in adjustable depth.
Application Environment
The product fulfills the requirements for type BF pursuant to
IEC/DIN EN 60601-1 and is used in operating rooms in sterile environ-
ments of explosion risk areas (such as areas with pure oxygen or anesthe-
sia gases).
2.2 Main functions and design characteristics
Electrical systems generally heat up during continual operation. It is
advised to give the system a break after use to cool down, as listed in the
table on operating mode.
Heating depends on the tool used and the load. After a certain number of
repetitions, the system should cool down. This procedure prevents the sys-
tem overheating as well as possible injury to the patient or user.
The user is responsible for the use and adherence to the pause sequence
described.
2.3 Indications
The type and area of application depend on the tool selected.
2.4 Absolute contraindications
The product is not licensed for use on the central nervous system or central
circulatory system.
Oscillation frequency min. 0 min-1 to max. 6 500 min-1
Operating mode Operation with non-periodic load and speed
changes (type S9 pursuant to IEC EN 60034-
1)
■60 second application, 60 second pause
■10 repetitions
■30 min cooling time
■Max. temperature 48 °C