Système de mini instruments Adtec
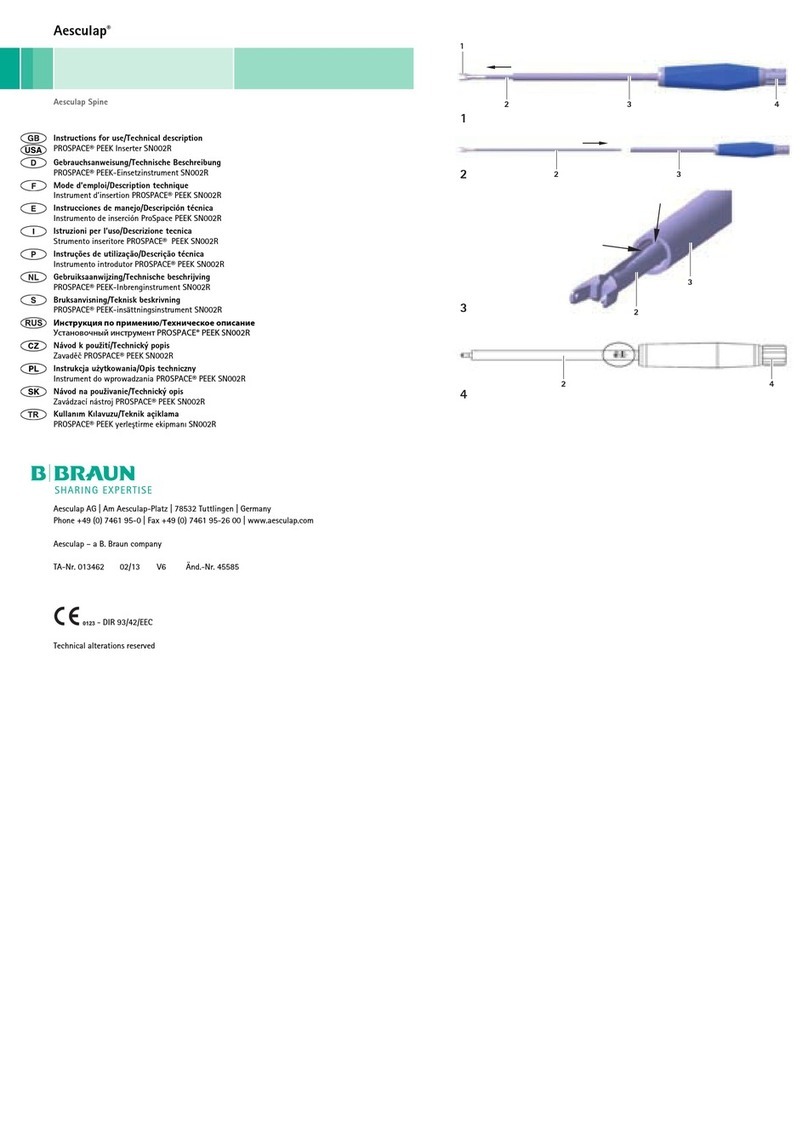
Légende
1Poignée (complète)
2Branche mobile de la poignée
3Broche HF
4Etoile tournante
5Levier d’actionnement
6Interrupteur (point d’huilage)
7Insert de mors (point d’huilage)
8Barre de compression
9Tube extérieur
Symboles sur le produit et emballage
Champ d’application
L’ensemble des disciplines d’endoscopie:
• Section, dissection, préhension de tissu
• Biopsie
• Sutures
Manipulation sûre et préparation
¾Confier le fonctionnement et l’utilisation de l’appareil et des accessoires uniquement à des personnes disposant
de la formation, des connaissances ou de l’expérience requises.
¾Lire, observer et conserver le mode d’emploi.
¾Utiliser le produit uniquement pour les fins prévues, voir Champ d’application.
¾Nettoyer minutieusement (à la main ou en machine) le produit neuf sortant d’usine après le retrait du
conditionnement de transport et avant la première stérilisation.
¾Conserver le produit neuf ou non utilisé dans un endroit sec, propre et protégé.
¾Avant chaque utilisation, procéder à un examen visuel du produit: absence de pièces lâches, tordues, brisées,
fissurées, usées et rompues.
¾Ne jamais utiliser un produit endommagé ou défectueux. Mettre immédiatement au rebut le produit
endommagé.
¾Remplacer immédiatement les pièces défectueuses par des pièces de rechange d'origine.
¾Pour éviter des dommages à l’extrémité de travail: introduire le produit avec précautions par le canal de travail
(p. ex. trocart).
¾Adapter la puissance de sortie HF à l’intervention. Tenir compte d’expériences ou de références cliniques.
¾Sélectionner une puissance de sortie HF aussi faible que possible.
¾Maintenir propres les surfaces de contact du produit au cours de l’opération. Essuyer avec un tampon humide
les résidus de tissus desséchés ou les liquides corporels.
Le produit est équipé côté connecteur du type de raccord suivant: broche élastique de 4 mm.
Le câble requis est indiqué dans nos prospectus.
La tension de référence des accessoires du produit est de 2 kVp.
La tension de référence des accessoires doit être supérieure ou égale à la tension de crête de sortie maximale avec
laquelle fonctionne le produit en association avec un appareil HF correspondant, un mode de fonctionnement/un
réglage correspondant (voir IEC/DIN EN 60601-2-2).
Pour éviter les brûlures par le courant HF:
¾Pendant l’activation HF, toujours conserver l’extrémité de travail du produit dans le champ de vision de
l’utilisateur.
¾Avant l’activation de l’appareil HF, contrôler que l’extrémité de travail du produit n’est pas en contact avec des
accessoires électriquement conducteurs.
¾Avant chaque utilisation, effectuer un contrôle visuel du produit: absence de détériorations et de modifications
de la surface de l'isolation.
¾Ne jamais poser le produit sur le patient ni directement à côté de lui.
¾En cas d’utilisation endoscopique ou laparoscopique d’accessoires, désactiver le mode d’activation automatique
de l’appareil HF.
¾Respecter le mode d’emploi de l’appareil HF.
Manipulation
¾Ouvrir et fermer la branche distale du mors:
Ouvrir et fermer la branche mobile de la poignée 2.
Poignée avec arrêt
¾Pour ouvrir le mécanisme à cran, presser le levier d’actionnement 5.
¾Pour activer le mécanisme à cran, relâcher le levier d’actionnement 5.
L’interrupteur 6sert à désactiver de façon permanente le mécanisme à cran.
¾Désenclencher le mécanisme à cran sur la poignée 1:
Position de l’interrupteur 6, voir I.
L’interrupteur 6s’engage dans la position avant.
¾Enclencher le mécanisme à cran sur la poignée 1:
Position de l’interrupteur 6, voir II.
L’interrupteur 6s’engage dans la position arrière.
Démontage
Remarque
Pour le démontage, voir aussi la série de figures supplémentaire
A
.
¾Désenclencher le mécanisme à cran sur la poignée 1avec arrêt:
Position de l’interrupteur 6, voir I.
¾Séparer la tige et la poignée 1:
Pousser l’étoile tournante 4vers l’arrière et la maintenir jusqu’à ce que la tige ait été retirée.
Tirer l’insert de mors 7en même temps que le tube extérieur 9hors de la poignée 1.
¾Démonter la tige:
Retirer le tube extérieur 9de l’insert de mors 7.
Tourner la barre de compression 8de 90° dans le sens inverse des aiguilles d’une montre et la tirer hors de
l’insert de mors 7.
Montage
Remarque
Pour le montage, voir aussi la série de photos supplémentaire
B
.
¾Désenclencher le mécanisme à cran sur la poignée 1avec arrêt:
Position de l’interrupteur 6, voir I.
¾Monter la tige:
– Pousser la barre de compression 8dans l’insert de mors 7et la tourner de 90° dans le sens des aiguilles d’une
montre.
– Pousser le tube extérieur 9jusqu’à la butée sur l’insert de mors 7.
¾Tenir d’une main la tige montée sur l’insert de mors 7.
¾Avec l’autre main, tenir la poignée 1par l’étoile tournante 4. La branche mobile 2doit pouvoir se mouvoir libre-
ment.
¾Pousser la tige dans la poignée 1. Veiller ce faisant à ce que l’encoche de la barre de compression 8soit dans
le même axe que le repère sur l’étoile tournante 4.
La branche mobile de la poignée 2se déplace vers le haut.
Dès que la butée est atteinte, la tige s’engage automatiquement.
¾Tester le fonctionnement de l’instrument en ouvrant et en fermant les branches du mors.
Procédé de traitement stérile validé
Remarque
En matière de traitement stérile, respecter les prescriptions légales nationales, les normes et directives nationales et
internationales ainsi que les propres dispositions adoptées en matière d’hygiène.
Remarque
Le traitement stérile en machine doit être préféré au nettoyage manuel du fait de résultats de nettoyage meilleurs et
plus fiables.
Remarque
On notera que la réussite du traitement stérile de ce produit médical ne peut être garantie qu’après validation
préalable du procédé de traitement stérile. La responsabilité en incombe à l’exploitant/au responsable du traitement
stérile.
Remarque
Pour des informations actuelles sur le traitement stérile, voir également l’Extranet Aesculap à l’adresse
www.aesculap-extra.net
Remarques générales
Pour éviter une contamination renforcée du plateau d’instruments garni, veiller lors de l’application à ce que les
instruments salis soient regroupés séparément et ne soient pas reposés sur le plateau d’instruments.
Les résidus opératoires incrustés ou fixés peuvent mettre obstacle au nettoyage ou le rendre inefficace et entraîner
une corrosion sur l’acier inoxydable. Un intervalle de 6 heures entre utilisation et nettoyage ne devrait par
conséquent pas être dépassé, de même qu’il ne faut pas appliquer de températures de prélavage fixantes >45 °C ni
utiliser de produits désinfectants fixants (substance active: aldéhyde, alcool).
Un surdosage du produit de neutralisation ou du détergent de base peut entraîner une agression chimique et/ou le
palissement et l’illisibilité visuelle ou mécanique de l’inscription laser sur l’acier inoxydable.
Sur l’acier inoxydable, les résidus contenant du chlore ou du chlorure, tels qu’ils sont contenus dans les résidus d’OP,
médicaments, sérum physiologique, eau de nettoyage, produits de décontamination et de stérilisation, entraînent
des dégâts dus à la corrosion (corrosion perforatrice, sous contrainte) et donc la dégradation des produits. Les résidus
sont éliminés par rinçage suffisamment abondant à l’eau déminéralisée et séchage consécutif.
Seuls doivent être utilisés pour le processus des produits chimiques contrôlés et validés (p. ex. agrément VAH/DGHM
ou FDA ou label CE) et recommandés par le fabricant des produits chimiques en termes de compatibilité avec les
matériaux. Toutes les prescriptions d’application du fabricant des produits chimiques relatives à la température, la
concentration et la durée d’action doivent être strictement respectées. Dans le cas contraire, les problèmes suivants
peuvent survenir:
• Détériorations de matériau telles que corrosion, fissures, cassures, vieillissement prématuré ou dilatation.
¾Ne pas utiliser pour le processus de produits chimiques qui entraînent sur les matières synthétiques des fissures
par contrainte ou une fragilisation.
¾Nettoyer le produit immédiatement après l’utilisation.
• Les incrustations sur les instruments HF sont détachées de manière particulièrement efficace et en douceur avec
un traitement par immersion d’env. 5 minutes dans une solution de H2O2à 3 %. Puis les résidus peuvent être
enlevés manuellement avec une brosse de dureté moyenne et/ou dans un bain à ultrasons. On procède ensuite
aux étapes usuelles suivantes du traitement stérile.
Pour des informations plus détaillées sur un retraitement hygiéniquement sûr qui ménage les matériaux et conserve
leur valeur aux produits, consulter www.a-k-i.org
¾En cas d’évacuation à l’état humide, utiliser un produit de nettoyage/décontamination adéquat. Pour éviter la
formation de mousse et une dégradation de l’efficacité des produits chimiques dans le processus: Avant le
nettoyage et la décontamination en machine, rincer abondamment le produit à l’eau courante.
Préparation au lieu d’utilisation
¾Démonter le produit immédiatement après usage suivant les instructions.
¾Ouvrir le produit muni d’une articulation.
¾Rincer les surfaces non visibles, p. ex. sur les produits présentant des fentes cachées, des lumières ou des
géométries complexes, de préférence à l’eau distillée, p. ex. avec une seringue à usage unique.
¾Retirer si possible complètement les résidus opératoires visibles avec un chiffon humide non pelucheux.
¾Présenter le produit sec au nettoyage et à la décontamination en container d’évacuation fermé dans un délai
de 6 h.
Préparation avant le nettoyage
¾Démonter le produit avant le nettoyage, voir Démontage.
Symbole Déclaration
Attention, symbole général de mise en garde
Attention, tenir compte des documents d’accompagnement
AVERTISSEMENT
Risque de blessure dû à l’inflammation ou à l’explosion de gaz inflammables!
Pendant l’utilisation conforme de l’appareil HF, des étincelles peuvent se produire.
¾Respecter les consignes de sécurité contenues dans le mode d’emploi de l’appareil
HF.
AVERTISSEMENT
Risques de lésions thermiques du patient/de l’utilisateur en cas de câbles insuffisam-
ment isolés des accessoires actifs!
¾Régler l’appareil HF de manière à ce que la tension de crête de sortie maximale
soit égale ou inférieure à la tension de référence des accessoires indiquée pour le
produit.
¾N’utiliser l’instrument qu’avec le tube extérieur isolé.
AVERTISSEMENT
Risque de blessure et/ou de dysfonctionnements!
¾Procéder à un contrôle du fonctionnement avant chaque utilisation.
AVERTISSEMENT
Risque de blessure en cas d’utilisation du produit en dehors du champ de visibilité!
¾Utiliser le produit uniquement sous contrôle visuel.